Reversibility of gastric mucosal lesions induced by sodium phosphate tablets and characterized by probe-based confocal laser endomicroscopy
- PMID: 26134776
- PMCID: PMC4423282
- DOI: 10.1055/s-0034-1377934
Reversibility of gastric mucosal lesions induced by sodium phosphate tablets and characterized by probe-based confocal laser endomicroscopy
Abstract
Background and study aims: Adequate bowel preparation is key for the optimal quality of colonoscopy. The sodium phosphate laxatives used for preparation may induce gastric injuries. However, in vivo studies monitoring the effects of sodium phosphate on the gastric mucosa are currently lacking. We aimed to characterize the effects of sodium phosphate tablets (Colokit®; Mayoly Spindler, Chatou, France) on the gastric mucosa in a large-animal model.
Methods: Fourteen anesthetized pigs were used for this study. Fundic mucosal sites were analyzed at 1.5, 24, and 72 hours after the endoscopically guided application of sodium phosphate tablets (NaPT) and placebo tablets (PlaT) and were compared with unexposed sites. Different mucosal parameters were assessed with white light endoscopy, probe-based confocal laser endomicroscopy (pCLE), histology, and ex vivo permeability measurements.
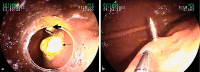
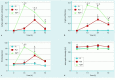
Results: At 90 minutes after the application of NaPT, significant increases in epithelial irregularity and crypt pit intensity were observed with pCLE. These microscopic lesions persisted at 24 hours but were resolved at 72 hours. In addition, white light endoscopy revealed local exanthema in 57 % of the animals at 1.5 hours after NaPT application. Such lesions were observed in 22 % of the pigs at 24 hours and disappeared at 72 hours after application. After 1.5 hours, PlaT induced a slight but significant increase in epithelial irregularity, as well as architectural scores that were significantly lower than the ones induced by NaPT and that disappeared after 72 hours.
Conclusions: The direct and prolonged gastric application of NaPT in pigs can induce acute superficial macroscopic and microscopic injuries that are reversible within 72 hours.
Conflict of interest statement
Figures



References
-
- Millan M S, Gross P, Manilich E. et al.Adenoma detection rate: the real indicator of quality in colonoscopy. Dis Colon Rectum. 2008;51:1217–1220. - PubMed
-
- Petersen B T. Quality measures and credentialing in gastrointestinal endoscopy. Curr Opin Gastroenterol. 2010;26:459–465. - PubMed
-
- Rex D K. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51:33–36. - PubMed
-
- Sanchez W, Harewood G C, Petersen B T. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol. 2004;99:1941–1945. - PubMed
-
- Segnan N, Patnick S, von Karsa L, Luxembourg: Publications Office of the European Union; 2010. European guidelines for quality assurance in colorectal cancer screening and diagnosis.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

