Acyl-CoA N-acyltransferase influences fertility by regulating lipid metabolism and jasmonic acid biogenesis in cotton
- PMID: 26134787
- PMCID: PMC4488762
- DOI: 10.1038/srep11790
Acyl-CoA N-acyltransferase influences fertility by regulating lipid metabolism and jasmonic acid biogenesis in cotton
Abstract
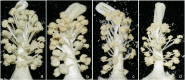
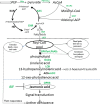
Cotton (Gossypium spp.) is an important economic crop and there is obvious heterosis in cotton, fertility has played an important role in this heterosis. However, the genes that exhibit critical roles in anther development and fertility are not well understood. Here, we report an acyl-CoA N-acyltransferase (EC2.3; GhACNAT) that plays a key role in anther development and fertility. Suppression of GhACNAT by virus-induced gene silencing in transgenic cotton (G. hirsutum L. cv. C312) resulted in indehiscent anthers that were full of pollen, diminished filaments and stamens, and plant sterility. We found GhACNAT was involved in lipid metabolism and jasmonic acid (JA) biosynthesis. The genes differentially expressed in GhACNAT-silenced plants and C312 were mainly involved in catalytic activity and transcription regulator activity in lipid metabolism. In GhACNAT-silenced plants, the expression levels of genes involved in lipid metabolism and jasmonic acid biosynthesis were significantly changed, the amount of JA in leaves and reproductive organs was significantly decreased compared with the amounts in C312. Treatments with exogenous methyl jasmonate rescued anther dehiscence and pollen release in GhACNAT-silenced plants and caused self-fertility. The GhACNAT gene may play an important role in controlling cotton fertility by regulating the pathways of lipid synthesis and JA biogenesis.
Figures




References
-
- Wilkins T. A., Mishra R. & Trolinder N. L. Agrobacterium-mediated transformation and regeneration of cotton. J. Food Agric. Environ. 2, 179–197 (2004).
-
- Wang K. et al.. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 44, 1098–1103 (2012). - PubMed
-
- Paterson A. H. et al.. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 492, 423–427 (2012). - PubMed
-
- Li F. et al.. Genome sequence of the cultivated cotton Gossypium arboreum. Nat. Genet. 46, 567–572 (2014). - PubMed
-
- Lange M., Aravinda L., Yellina, Svetlana O. & Annette B. Virus-Induced Gene Silencing (VIGS) in plants: an overview of target apecies and the virus-derived vector systems. Methods in Mol. Biol. 975, 1–14 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

