Serine Arginine-Rich Splicing Factor 1 (SRSF1) Contributes to the Transcriptional Activation of CD3ζ in Human T Cells
- PMID: 26134847
- PMCID: PMC4489909
- DOI: 10.1371/journal.pone.0131073
Serine Arginine-Rich Splicing Factor 1 (SRSF1) Contributes to the Transcriptional Activation of CD3ζ in Human T Cells
Abstract
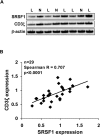
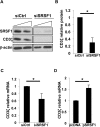
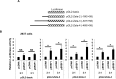
T lymphocytes from many patients with systemic lupus erythematosus (SLE) express decreased levels of the T cell receptor (TCR)-associated CD3 zeta (ζ) signaling chain, a feature directly linked to their abnormal phenotype and function. Reduced mRNA expression partly due to defective alternative splicing, contributes to the reduced expression of CD3ζ chain. We previously identified by oligonucleotide pulldown and mass spectrometry approaches, the serine arginine-rich splicing factor 1 (SRSF1) binding to the 3' untranslated region (UTR) of CD3ζ mRNA. We showed that SRSF1 regulates alternative splicing of the 3'UTR of CD3ζ to promote expression of the normal full length 3`UTR over an unstable splice variant in human T cells. In this study we show that SRSF1 regulates transcriptional activation of CD3ζ. Specifically, overexpression and silencing of SRSF1 respectively increases and decreases CD3ζ total mRNA and protein expression in Jurkat and primary T cells. Using promoter-luciferase assays, we show that SRSF1 enhances transcriptional activity of the CD3ζ promoter in a dose dependent manner. Chromatin immunoprecipitation assays show that SRSF1 is recruited to the CD3ζ promoter. These results indicate that SRSF1 contributes to transcriptional activation of CD3ζ. Thus our study identifies a novel mechanism whereby SRSF1 regulates CD3ζ expression in human T cells and may contribute to the T cell defect in SLE.
Conflict of interest statement
Figures


References
-
- Nambiar MP, Enyedy EJ, Warke VG, Krishnan S, Dennis G, Kammer GM, et al. Polymorphisms/mutations of TCR-zeta-chain promoter and 3' untranslated region and selective expression of TCR zeta-chain with an alternatively spliced 3' untranslated region in patients with systemic lupus erythematosus. Journal of autoimmunity. 2001;16(2):133–42. Epub 2001/03/15. 10.1006/jaut.2000.0475 S0896-8411(00)90475-X [pii]. ; PubMed Central PMCID: PMCPMC Journal—In Process. - DOI - PubMed
-
- Brundula V, Rivas LJ, Blasini AM, Paris M, Salazar S, Stekman IL, et al. Diminished levels of T cell receptor zeta chains in peripheral blood T lymphocytes from patients with systemic lupus erythematosus. Arthritis and rheumatism. 1999;42(9):1908–16. Epub 1999/10/08. 10.1002/1529-0131(199909)42:9<1908::AID-ANR17>3.0.CO;2-7 ; PubMed Central PMCID: PMCPMC Journal—In Process. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

