IsaB Inhibits Autophagic Flux to Promote Host Transmission of Methicillin-Resistant Staphylococcus aureus
- PMID: 26134948
- PMCID: PMC4641007
- DOI: 10.1038/jid.2015.254
IsaB Inhibits Autophagic Flux to Promote Host Transmission of Methicillin-Resistant Staphylococcus aureus
Abstract
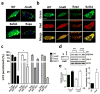
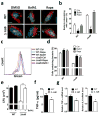
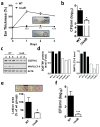
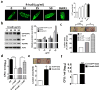
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a major nosocomial pathogen that is widespread in both health-care facilities and in the community at large, as a result of direct host-to-host transmission. Several virulence factors are associated with pathogen transmission to naive hosts. Immunodominant surface antigen B (IsaB) is a virulence factor that helps Staphylococcus aureus to evade the host defense system. However, the mechanism of IsaB on host transmissibility remains unclear. We found that IsaB expression was elevated in transmissible MRSA. Wild-type isaB strains inhibited autophagic flux to promote bacterial survival and elicit inflammation in THP-1 cells and mouse skin. MRSA isolates with increased IsaB expression showed decreased autophagic flux, and the MRSA isolate with the lowest IsaB expression showed increased autophagic flux. In addition, recombinant IsaB rescued the virulence of the isaB deletion strain and increased the group A streptococcus (GAS) virulence in vivo. Together, these results reveal that IsaB diminishes autophagic flux, thereby allowing MRSA to evade host degradation. These findings suggest that IsaB is a suitable target for preventing or treating MRSA infection.
Conflict of interest statement
The authors state no conflict of interest.
Figures


References
-
- Amano A, Nakagawa I, Yoshimori T. Autophagy in innate immunity against intracellular bacteria. Journal of biochemistry. 2006;140:161–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

