Neutrophil Depletion Attenuates Placental Ischemia-Induced Hypertension in the Rat
- PMID: 26135305
- PMCID: PMC4509576
- DOI: 10.1371/journal.pone.0132063
Neutrophil Depletion Attenuates Placental Ischemia-Induced Hypertension in the Rat
Abstract
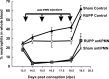
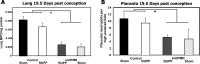
Preeclampsia is characterized by reduced placental perfusion with placental ischemia and hypertension during pregnancy. Preeclamptic women also exhibit a heightened inflammatory state and greater number of neutrophils in the vasculature compared to normal pregnancy. Since neutrophils are associated with tissue injury and inflammation, we hypothesized that neutrophils are critical to placental ischemia-induced hypertension and fetal demise. Using the reduced uteroplacental perfusion pressure (RUPP) model of placental ischemia-induced hypertension in the rat, we determined the effect of neutrophil depletion on blood pressure and fetal resorptions. Neutrophils were depleted with repeated injections of polyclonal rabbit anti-rat polymorphonuclear leukocyte (PMN) antibody (antiPMN). Rats received either antiPMN or normal rabbit serum (Control) on 13.5, 15.5, 17.5, and 18.5 days post conception (dpc). On 14.5 dpc, rats underwent either Sham surgery or clip placement on ovarian arteries and abdominal aorta to reduce uterine perfusion pressure (RUPP). On 18.5 dpc, carotid arterial catheters were placed and mean arterial pressure (MAP) was measured on 19.5 dpc. Neutrophil-depleted rats had reduced circulating neutrophils from 14.5 to 19.5 dpc compared to Control, as well as decreased neutrophils in lung and placenta on 19.5 dpc. MAP increased in RUPP Control vs Sham Control rats, and neutrophil depletion attenuated this increase in MAP in RUPP rats without any effect on Sham rats. The RUPP-induced increase in fetal resorptions and complement activation product C3a were not affected by neutrophil depletion. Thus, these data are the first to indicate that neutrophils play an important role in RUPP hypertension and that cells of the innate immune system may significantly contribute to pregnancy-induced hypertension.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

