Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning
- PMID: 26135716
- PMCID: PMC4648295
- DOI: 10.1002/hipo.22488
Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning
Abstract
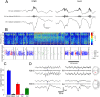
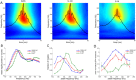
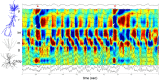
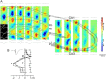
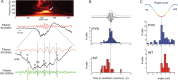
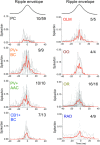
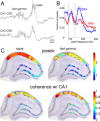
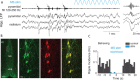
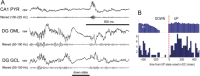
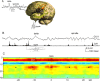
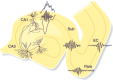
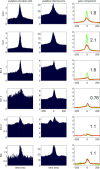
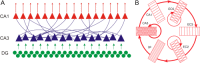
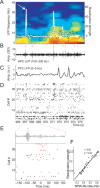
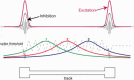
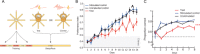
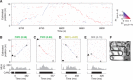
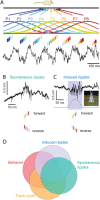
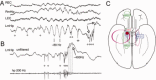
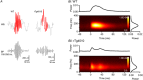
Sharp wave ripples (SPW-Rs) represent the most synchronous population pattern in the mammalian brain. Their excitatory output affects a wide area of the cortex and several subcortical nuclei. SPW-Rs occur during "off-line" states of the brain, associated with consummatory behaviors and non-REM sleep, and are influenced by numerous neurotransmitters and neuromodulators. They arise from the excitatory recurrent system of the CA3 region and the SPW-induced excitation brings about a fast network oscillation (ripple) in CA1. The spike content of SPW-Rs is temporally and spatially coordinated by a consortium of interneurons to replay fragments of waking neuronal sequences in a compressed format. SPW-Rs assist in transferring this compressed hippocampal representation to distributed circuits to support memory consolidation; selective disruption of SPW-Rs interferes with memory. Recently acquired and pre-existing information are combined during SPW-R replay to influence decisions, plan actions and, potentially, allow for creative thoughts. In addition to the widely studied contribution to memory, SPW-Rs may also affect endocrine function via activation of hypothalamic circuits. Alteration of the physiological mechanisms supporting SPW-Rs leads to their pathological conversion, "p-ripples," which are a marker of epileptogenic tissue and can be observed in rodent models of schizophrenia and Alzheimer's Disease. Mechanisms for SPW-R genesis and function are discussed in this review.
Keywords: epilepsy; imagining; learning; memory; planning.
© 2015 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Figures






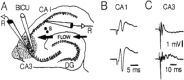








References
-
- Abeles M, Bergman H, Margalit E, Vaadia E. 1993. Spatiotemporal firing patterns in the frontal cortex of behaving monkeys. J Neurophysiol 70:1629–1638. - PubMed
-
- Abraham WC, Gustafsson B, Wigstrom H. 1986. Single high strength afferent volleys can produce long‐term potentiation in the hippocampus in vitro. Neurosci Lett 70:217–222. - PubMed
-
- Adey WR, Kado RT, Rhodes JM. 1963. Sleep: Cortical and subcortical recordings in the chimpanzee. Science 141:932–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

