IL-1 Receptor Antagonist Treatment Aggravates Staphylococcal Septic Arthritis and Sepsis in Mice
- PMID: 26135738
- PMCID: PMC4489902
- DOI: 10.1371/journal.pone.0131645
IL-1 Receptor Antagonist Treatment Aggravates Staphylococcal Septic Arthritis and Sepsis in Mice
Abstract
Background: Interleukin-1 receptor antagonist (IL-1Ra) is the primary therapy against autoinflammatory syndromes with robust efficacy in reducing systemic inflammation and associated organ injury. However, patients receiving IL-1Ra might be at increased risk of acquiring serious infections.
Aims: To study whether IL-1Ra treatment deteriorates Staphylococcus aureus (S. aureus) septic arthritis and sepsis in mice.
Method: NMRI mice were treated with anakinra (IL-1Ra) daily for 7 days before intravenous inoculation with S. aureus strain Newman in both arthritogenic and lethal doses. The clinical course of septic arthritis, histopathological and radiological changes of the joints, as well as the mortality were compared between IL-1Ra treated and control groups.
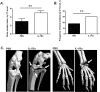
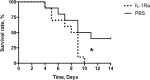
Results: IL-1Ra treated mice developed more frequent and severe clinical septic arthritis. Also, the frequency of polyarthritis was significantly higher in the mice receiving IL-1Ra therapy. In line with the data from clinical arthritis, both histological and radiological signs of septic arthritis were more pronounced in IL-1Ra treated group compared to controls. Importantly, the mortality of IL-1Ra treated mice was significantly higher than PBS treated controls.
Conclusion: IL-1Ra treatment significantly aggravated S. aureus induced septic arthritis and increased the mortality in these mice.
Conflict of interest statement
Figures

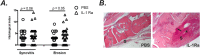
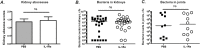

References
-
- Cao Y, Jiao Y, Wang L, Huang Y, Postlethwaite A, Stuart J, et al. Anakinra as an interleukin 1 receptor antagonist, complicated genetics and molecular impacts—from the point of view of mouse genomics. Int Immunopharmacol. 2012;13(1):28–36. Epub 2012/03/20. doi: S1567-5769(12)00066-5 [pii] 10.1016/j.intimp.2012.02.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

