Overexpression of connexin 43 reduces melanoma proliferative and metastatic capacity
- PMID: 26135897
- PMCID: PMC4506378
- DOI: 10.1038/bjc.2015.162
Overexpression of connexin 43 reduces melanoma proliferative and metastatic capacity
Erratum in
-
Overexpression of connexin 43 reduces melanoma proliferative and metastatic capacity.Br J Cancer. 2016 Oct 25;115(9):e14. doi: 10.1038/bjc.2016.296. Epub 2016 Sep 22. Br J Cancer. 2016. PMID: 27657340 Free PMC article. No abstract available.
Abstract
Background: Alterations in connexin 43 (Cx43) expression and/or gap junction (GJ)-mediated intercellular communication are implicated in cancer pathogenesis. Herein, we have investigated the role of Cx43 in melanoma cell proliferation and apoptosis sensitivity in vitro, as well as metastatic capability and tumour growth in vivo.
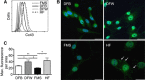
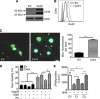
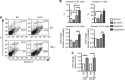
Methods: Connexin 43 expression levels, GJ coupling and proliferation rates were analysed in four different human melanoma cell lines. Furthermore, tumour growth and lung metastasis of high compared with low Cx43-expressing FMS cells were evaluated in vivo using a melanoma xenograft model.
Results: Specific inhibition of Cx43 channel activity accelerated melanoma cell proliferation, whereas overexpression of Cx43 increased GJ coupling and reduced cell growth. Moreover, Cx43 overexpression in FMS cells increased basal and tumour necrosis factor-α-induced apoptosis and resulted in decreased melanoma tumour growth and lower number and size of metastatic foci in vivo.
Conclusions: Our findings reveal an important role for Cx43 in intrinsically controlling melanoma growth, death and metastasis, and emphasise the potential use of compounds that selectively enhance Cx43 expression on melanoma in the future chemotherapy and/or immunotherapy protocols.
Figures


References
-
- Becker D, Ciantar D, Catsicas M, Pearson R, Mobbs P. Use of pIRES vectors to express EGFP and connexin constructs in studies of the role of gap junctional communication in the early development of the chick retina and brain. Cell Commun Adhes. 2001;8:355–359. - PubMed
-
- Carette D, Gilleron J, Chevallier D, Segretain D, Pointis G. Connexin a check-point component of cell apoptosis in normal and physiological conditions. Biochimie. 2014;101:1–9. - PubMed
-
- Conklin CMJ, Bechberger JF, MacFabe D, Guthrie N, Kurowska E, Naus CC. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis. 2007;28:93–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

