Ocular Fluid As a Replacement for Serum in Cell Cryopreservation Media
- PMID: 26135924
- PMCID: PMC4489643
- DOI: 10.1371/journal.pone.0131291
Ocular Fluid As a Replacement for Serum in Cell Cryopreservation Media
Abstract
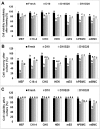
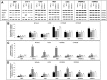
Cryostorage is of immense interest in biomedical research, especially for stem cell-based therapies and fertility preservation. Several protocols have been developed for efficient cryopreservation of cells and tissues, and a combination of dimethyl sulfoxide (DMSO) and fetal bovine serum (FBS) is commonly used. However, there is a need for an alternative to FBS because of ethical reasons, high cost, and risk of contamination with blood-borne diseases. The objective of the present study was to examine the possibility of using buffalo (Bubalus bubalis) ocular fluid (BuOF) to replace FBS in cryomedia. Frozen-thawed cells, which were cryopreserved in a cryomedia with BuOF, were assessed for viability, early and late apoptosis, and proliferation. Three cell lines (CHO, HEK, and C18-4), mouse embryonic stem (mES) cells, and primary cells, such as mouse embryonic fibroblast (MEF) cells, human peripheral blood mononuclear cells (hPBMCs), and mouse bone marrow cells (mBMCs), were cryopreserved in cryomedia containing 10% DMSO (D10) with 20% FBS (D10S20) or D10 with 20% BuOF (D10O20). For all three cell lines and mES cells cryopreserved in either D10S20 or D10O20, thawed cells showed no difference in cell viability or cell recovery. Western blot analysis of frozen-thawed-cultured cells revealed that the expression of Annexin V and proliferating cell nuclear antigen (PCNA) proteins, and the ratio of BAX/BCL2 proteins were similar in all three cell lines, mES cells, and hPBMCs cryopreserved in D10S20 and D10O20. However, initial cell viability, cell recovery after culture, and PCNA expression were significantly lower in MEF cells, and the BAX/BCL2 protein ratio was elevated in mBMCs cryopreserved in D10O20. Biochemical and proteomic analysis of BuOF showed the presence of several components that may have roles in imparting the cryoprotective property of BuOF. These results encourage further research to develop an efficient serum-free cryomedia for several cell types using BuOF.
Conflict of interest statement
Figures
References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

