Radiosensitization and downregulation of heterogeneous nuclear ribonucleoprotein K (hnRNP K) upon inhibition of mitogen/extracellular signal-regulated kinase (MEK) in malignant melanoma cells
- PMID: 26136337
- PMCID: PMC4627300
- DOI: 10.18632/oncotarget.3935
Radiosensitization and downregulation of heterogeneous nuclear ribonucleoprotein K (hnRNP K) upon inhibition of mitogen/extracellular signal-regulated kinase (MEK) in malignant melanoma cells
Abstract
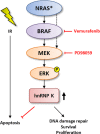
Background: Heterogeneous nuclear ribonucleoprotein K (hnRNP K) is an important cofactor in the p53-mediated DNA damage response pathway upon ionizing radiation (IR) and exerts anti-apoptotic effects also independent of p53 pathway activation. Furthermore, hnRNP K is overexpressed in various neoplasms including malignant melanoma (MM). Here, we investigate the role of hnRNP K in the radioresistance of MM cells.
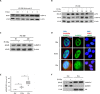
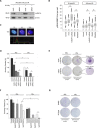
Methods and results: Our results show cytoplasmic expression of hnRNP K in human MM surgical specimens, but not in benign nevi, and a quick dose- and time-dependent upregulation in response to IR accompanied by cytoplasmic redistribution of the protein in the IPC-298 cellular tumor model carrying an activating NRAS mutation (p.Q61L). SiRNA-based knockdown of hnRNP K induced a delayed decline in γH2AX/53BP1-positive DNA repair foci upon IR. Pharmacological interference with MAPK signaling abrogated ERK phosphorylation, diminished cellular hnRNP K levels, impaired γH2AX/53BP1-foci repair and proliferative capability and increased apoptosis comparable to the observed hnRNP K knockdown phenotype in IPC-298 cells.
Conclusions: Our results indicate that pharmacological interference with MAPK signaling increases vulnerability of NRAS-mutant malignant melanoma cells to ionizing radiation along with downregulation of endogenous hnRNP K and point towards a possible use for combined MEK inhibition and localized radiation therapy of MM in the NRAS-mutant setting where BRAF inhibitors offer no clinical benefit.
Keywords: MEK inhibition; NRAS; melanoma; nRNP K; radiotherapy.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


References
-
- Rao NG, Yu HH, Trotti A, 3rd, Sondak VK. The role of radiation therapy in the management of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:115–31. - PubMed
-
- Pflugfelder A, Kochs C, Blum AM, Capellaro M, Czeschik C, Dettenborn T, Dill D, Dippel E, Eigentler T, Feyer P, Follmann M, Frerich B, Ganten MK, Gartner J, Gutzmer R, et al. Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma”. J Dtsch Dermatol Ges. 2013;11:1–116. 1–126. - PubMed
-
- Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA, Carruthers S, Coventry BJ, Babington S, Duprat J, Hoekstra HJ, Thompson JF. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13:589–97. - PubMed
-
- Moumen A, Masterson P, O'Connor MJ, Jackson SP, hnRNP K. an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123:1065–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

