Facing and Overcoming Sensitivity Challenges in Biomolecular NMR Spectroscopy
- PMID: 26136394
- PMCID: PMC4943876
- DOI: 10.1002/anie.201410653
Facing and Overcoming Sensitivity Challenges in Biomolecular NMR Spectroscopy
Abstract
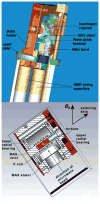
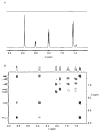
In the Spring of 2013, NMR spectroscopists convened at the Weizmann Institute in Israel to brainstorm on approaches to improve the sensitivity of NMR experiments, particularly when applied in biomolecular settings. This multi-author interdisciplinary Review presents a state-of-the-art description of the primary approaches that were considered. Topics discussed included the future of ultrahigh-field NMR systems, emerging NMR detection technologies, new approaches to nuclear hyperpolarization, and progress in sample preparation. All of these are orthogonal efforts, whose gains could multiply and thereby enhance the sensitivity of solid- and liquid-state experiments. While substantial advances have been made in all these areas, numerous challenges remain in the quest of endowing NMR spectroscopy with the sensitivity that has characterized forms of spectroscopies based on electrical or optical measurements. These challenges, and the ways by which scientists and engineers are striving to solve them, are also addressed.
Keywords: NMR probeheads; NMR spectroscopy; nuclear hyperpolarization; sensitivity enhancement; ultrahigh magnetic fields.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures











References
-
- Williamson MP, Havel TF, Wüthrich K. J Mol Biol. 1985;182:295–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

