Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction
- PMID: 26136410
- PMCID: PMC4435095
- DOI: 10.1002/jcsm.12009
Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction
Abstract
Background: The majority of cancer patients loses weight and becomes malnourished during the course of their disease. Metabolic alterations and reduced immune competence lead to wasting and an increased risk of infectious complications. In the present study, the effect of a nutritionally complete medical food, which is high in protein and leucine and enriched with fish oil and specific oligosaccharides, was investigated on immune function, nutritional status, and inflammation in patients with esophageal cancer and compared with routine care.
Methods: In this exploratory double-blind study, 64 newly diagnosed esophageal cancer patients were randomized. All patients received dietary counselling and dietary advice. In the Active group, all patients received the specific medical food for 4 weeks before the start of anticancer therapy. In the routine care control arm, patients with <5% weight loss received a non-caloric placebo product, and patients with weight loss ≥5% received an iso-caloric control product to secure blinding of the study. The required study parameters of body weight and performance status were recorded at baseline and after 4 weeks of nutritional intervention, and patients were asked to complete quality of life questionnaires. In addition, blood samples were taken for the measurement of several immune, nutritional, and safety-parameters.
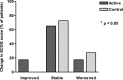
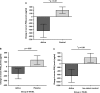
Results: No effect of the specific nutritional intervention could be detected on ex vivo stimulations of blood mononuclear cells. By contrast, body weight was significantly increased (P < 0.05) and ECOG performance status was improved after intervention with the specific medical food (P < 0.05). In addition, serum Prostaglandin E2 (PGE2) levels were significantly decreased in the specific medical food group and increased in the control group (P = 0.002).
Conclusions: Nutritional intervention with the specific medical food significantly increased body weight and improved performance status compared with routine care in newly diagnosed esophageal cancer patients. This effect was accompanied by significantly reduced serum PGE2 levels.
Keywords: Body weight; Cancer; Clinical; Medical food; Nutrition; PGE2.
© 2015 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society of Sarcopenia, Cachexia and Wasting Disorders.
Figures

Similar articles
-
Rapid EPA and DHA incorporation and reduced PGE2 levels after one week intervention with a medical food in cancer patients receiving radiotherapy, a randomized trial.Clin Nutr. 2013 Jun;32(3):338-45. doi: 10.1016/j.clnu.2012.09.009. Epub 2012 Sep 28. Clin Nutr. 2013. PMID: 23123043 Clinical Trial.
-
A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial.Cancer. 2013 Sep 15;119(18):3343-53. doi: 10.1002/cncr.28197. Epub 2013 Jun 13. Cancer. 2013. PMID: 23765693 Clinical Trial.
-
Weight loss and resting energy expenditure in male patients with newly diagnosed esophageal cancer.Nutrition. 2013 Nov-Dec;29(11-12):1310-4. doi: 10.1016/j.nut.2013.04.010. Epub 2013 Sep 4. Nutrition. 2013. PMID: 24012284
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
[Simple obesity in children. A study on the role of nutritional factors].Med Wieku Rozwoj. 2006 Jan-Mar;10(1):3-191. Med Wieku Rozwoj. 2006. PMID: 16733288 Review. Polish.
Cited by
-
Elevated Serum AA/EPA Ratio as a Predictor of Skeletal Muscle Depletion in Cachexic Patients with Advanced Gastro-intestinal Cancers.In Vivo. 2017 Sep-Oct;31(5):1003-1009. doi: 10.21873/invivo.11161. In Vivo. 2017. PMID: 28882973 Free PMC article.
-
Oodles of opportunities: the Journal of Cachexia, Sarcopenia and Muscle in 2017.J Cachexia Sarcopenia Muscle. 2017 Oct;8(5):675-680. doi: 10.1002/jcsm.12247. J Cachexia Sarcopenia Muscle. 2017. PMID: 29076661 Free PMC article. No abstract available.
-
Cancer cachexia: A scoping review on non-pharmacological interventions.Asia Pac J Oncol Nurs. 2024 Mar 12;11(5):100438. doi: 10.1016/j.apjon.2024.100438. eCollection 2024 May. Asia Pac J Oncol Nurs. 2024. PMID: 38774537 Free PMC article.
-
Dose-Dependent Impacts of Omega-3 Fatty Acids Supplementation on Anthropometric Variables in Patients With Cancer: Results From a Systematic Review and Meta-Analysis of Randomized Clinical Trials.Clin Nutr Res. 2024 Jul 29;13(3):186-200. doi: 10.7762/cnr.2024.13.3.186. eCollection 2024 Jul. Clin Nutr Res. 2024. PMID: 39165286 Free PMC article. Review.
-
Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade.Nutrients. 2021 Aug 12;13(8):2766. doi: 10.3390/nu13082766. Nutrients. 2021. PMID: 34444926 Free PMC article. Review.
References
-
- Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol. 1999;8:133–141. - PubMed
-
- Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 2000;34:137–168. - PubMed
-
- Van Bokhorst-de Van der Schueren MA. Nutritional support strategies for malnourished cancer patients. Eur J Oncol Nurs. 2005;9:S74–S83. - PubMed
-
- Argiles JM. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S39–S50. - PubMed
-
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO, Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

