Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines
- PMID: 26136413
- PMCID: PMC4435098
- DOI: 10.1002/jcsm.12006
Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines
Abstract
Background: Chronic heart failure (CHF) is commonly associated with muscle atrophy and increased inflammation. Irisin, a myokine proteolytically processed by the fibronectin type III domain containing 5 (FNDC5) gene and suggested to be Peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α activated, modulates the browning of adipocytes and is related to muscle mass. Therefore, we investigated whether skeletal muscle FNDC5 expression in CHF was reduced and if this was mediated by inflammatory cytokines and/or angiotensin II (Ang-II).
Methods: Skeletal muscle FNDC5 mRNA/protein and PGC-1α mRNA expression (arbitrary units) were analysed in: (i) rats with ischemic cardiomyopathy; (ii) mice injected with tumour necrosis factor-α (TNF-α) (24 h); (iii) mice infused with Ang-II (4 weeks); and (iv) C2C12 myotubes exposed to recombinant cytokines or Ang-II. Circulating TNF-α, Ang-II, and irisin was measured by ELISA.
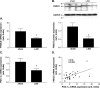
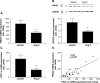
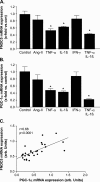
Results: Ischemic cardiomyopathy reduced significantly FNDC5 protein (1.3 ± 0.2 vs. 0.5 ± 0.1) and PGC-1α mRNA expression (8.2 ± 1.5 vs. 4.7 ± 0.7). In vivo TNF-α and Ang-II reduced FNDC5 protein expression by 28% and 45%, respectively. Incubation of myotubes with TNF-α, interleukin-1ß, or TNF-α/interleukin-1ß reduced FNDC5 protein expression by 47%, 37%, or 57%, respectively, whereas Ang-II had no effect. PGC-1α was linearly correlated to FNDC5 in all conditions. In CHF, animals circulating TNF-α and Ang-II were significantly increased, whereas irisin was significantly reduced. A negative correlation between circulating TNF-α and irisin was evident.
Conclusion: A reduced expression of skeletal muscle FNDC5 in ischemic cardiomyopathy is likely modulated by inflammatory cytokines and/or Ang-II via the down-regulation of PGC-1α. This may act as a protective mechanism either by slowing the browning of adipocytes and preserving energy homeostasis or by regulating muscle atrophy.
Keywords: Angiotensin II; Chronics heart failure; Cytokines; FNDC5; Irisin; Skeletal muscle.
© 2015 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society of Cachexia, Sarcopenia and Wasting Disorders.
Figures



References
-
- Okita K, Kinugawa S, Tsutsui H. Exercise intolerance in chronic heart failure – skeletal muscle dysfunction and potential therapies. Circ J. 2013;77:293–300. - PubMed
-
- Gielen S, Adams V, Möbius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Antiinflammatory effects of exercise training in the skeletal muscle of patients with Chronic Heart Failure. J Am Coll Cardiol. 2003;42:861–868. - PubMed
-
- Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. - PubMed
-
- Li YP, Schwartz RJ, Wadell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF.kB activation in response to tumor necrosis factor a. FASEB J. 1998;12:871–880. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

