Differences in food intake of tumour-bearing cachectic mice are associated with hypothalamic serotonin signalling
- PMID: 26136415
- PMCID: PMC4435100
- DOI: 10.1002/jcsm.12008
Differences in food intake of tumour-bearing cachectic mice are associated with hypothalamic serotonin signalling
Abstract
Background: Anorexia is a common symptom among cancer patients and contributes to malnutrition and strongly impinges on quality of life. Cancer-induced anorexia is thought to be caused by an inability of food intake-regulating systems in the hypothalamus to respond adequately to negative energy balance during tumour growth. Here, we show that this impaired response of food-intake control is likely to be mediated by altered serotonin signalling and by failure in post-transcriptional neuropeptide Y (NPY) regulation.
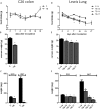
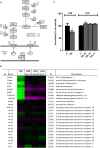
Methods: Two tumour cachectic mouse models with different food intake behaviours were used: a C26-colon adenocarcinoma model with increased food intake and a Lewis lung carcinoma model with decreased food intake. This contrast in food intake behaviour between tumour-bearing (TB) mice in response to growth of the two different tumours was used to distinguish between processes involved in cachexia and mechanisms that might be important in food intake regulation. The hypothalamus was used for transcriptomics (affymetrix chips).
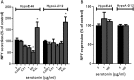
Results: In both models, hypothalamic expression of orexigenic NPY was significantly higher compared with controls, suggesting that this change does not directly reflect food intake status but might be linked to negative energy balance in cachexia. Expression of genes involved in serotonin signalling showed to be different between C26-TB mice and Lewis lung carcinoma-TB mice and was inversely associated with food intake. In vitro, using hypothalamic cell lines, serotonin repressed neuronal hypothalamic NPY secretion while not affecting messenger NPY expression, suggesting that serotonin signalling can interfere with NPY synthesis, transport, or secretion.
Conclusions: Altered serotonin signalling is associated with changes in food intake behaviour in cachectic TB mice. Serotonins' inhibitory effect on food intake under cancer cachectic conditions is probably via affecting the NPY system. Therefore, serotonin regulation might be a therapeutic target to prevent the development of cancer-induced eating disorders.
Keywords: Anorexia; Cachexia; Cancer; Neuropeptide Y; Serotonin.
© 2015 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society of Cachexia, Sarcopenia and Wasting Disorders.
Figures

Similar articles
-
Hypothalamic food intake regulation in a cancer-cachectic mouse model.J Cachexia Sarcopenia Muscle. 2014 Jun;5(2):159-69. doi: 10.1007/s13539-013-0121-y. Epub 2013 Nov 13. J Cachexia Sarcopenia Muscle. 2014. PMID: 24222472 Free PMC article.
-
Hypothalamic integration of immune function and metabolism.Prog Brain Res. 2006;153:367-405. doi: 10.1016/S0079-6123(06)53022-5. Prog Brain Res. 2006. PMID: 16876587 Free PMC article. Review.
-
Normalization of hypothalamic serotonin (5-HT 1B) receptor and NPY in cancer anorexia after tumor resection: an immunocytochemical study.Neurosci Lett. 2005 Aug 5;383(3):322-7. doi: 10.1016/j.neulet.2005.04.031. Neurosci Lett. 2005. PMID: 15955429
-
Omega-3 fatty acids improve appetite in cancer anorexia, but tumor resecting restores it.Surgery. 2006 Feb;139(2):202-8. doi: 10.1016/j.surg.2005.08.001. Surgery. 2006. PMID: 16455329
-
Hypothalamic inflammation and food intake regulation during chronic illness.Peptides. 2016 Mar;77:60-6. doi: 10.1016/j.peptides.2015.06.011. Epub 2015 Jul 6. Peptides. 2016. PMID: 26158772 Review.
Cited by
-
Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature.Nutrients. 2022 Jul 9;14(14):2824. doi: 10.3390/nu14142824. Nutrients. 2022. PMID: 35889781 Free PMC article. Review.
-
Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia.J Cachexia Sarcopenia Muscle. 2020 Jun;11(3):619-635. doi: 10.1002/jcsm.12528. Epub 2020 Mar 6. J Cachexia Sarcopenia Muscle. 2020. PMID: 32142217 Free PMC article. Review.
-
Anorexia of ageing: a key component in the pathogenesis of both sarcopenia and cachexia.J Cachexia Sarcopenia Muscle. 2017 Aug;8(4):523-526. doi: 10.1002/jcsm.12192. Epub 2017 Apr 27. J Cachexia Sarcopenia Muscle. 2017. PMID: 28452130 Free PMC article.
-
Casting the net broader to confirm our imaginations: the long road to treating wasting disorders.J Cachexia Sarcopenia Muscle. 2017 Dec;8(6):870-880. doi: 10.1002/jcsm.12256. J Cachexia Sarcopenia Muscle. 2017. PMID: 29168628 Free PMC article.
-
The atypical β-blocker S-oxprenolol reduces cachexia and improves survival in a rat cancer cachexia model.J Cachexia Sarcopenia Muscle. 2023 Feb;14(1):653-660. doi: 10.1002/jcsm.13116. Epub 2022 Nov 8. J Cachexia Sarcopenia Muscle. 2023. PMID: 36346141 Free PMC article.
References
-
- Sarhill N, Mahmoud F, Walsh D, Nelson KA, Komurcu S, Davis M, LeGrand S, Abdullah O, Rybicki L. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer. 2003;11:652–659. - PubMed
-
- Okusaka T, Okada S, Ishii H, Ikeda M, Kosakamoto H, Yoshimori M. Prognosis of advanced pancreatic cancer patients with reference to calorie intake. Nutr Cancer. 1998;32:55–58. - PubMed
-
- Tamburini M, Brunelli C, Rosso S, Ventafridda V. Prognostic value of quality of life scores in terminal cancer patients. J Pain Symptom Manage. 1996;11:32–41. - PubMed
-
- Laviano A, Inui A, Meguid MM, Molfino A, Conte C, Rossi Fanelli F. NPY and brain monoamines in the pathogenesis of cancer anorexia. Nutrition. 2008;24:802–805. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

