Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane
- PMID: 26136560
- PMCID: PMC4556887
- DOI: 10.1152/ajprenal.00631.2014
Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane
Abstract
Phosphatidylinositol bisphosphate (PIP2) regulates epithelial sodium channel (ENaC) open probability. In turn, myristoylated alanine-rich C kinase substrate (MARCKS) protein or MARCKS-like protein 1 (MLP-1) at the plasma membrane regulates the delivery of PIP2 to ENaC. MARCKS and MLP-1 are regulated by changes in cytosolic calcium; increasing calcium promotes dissociation of MARCKS from the membrane, but the calcium-regulatory mechanisms are unclear. However, it is known that increased intracellular calcium can activate calmodulin and we show that inhibition of calmodulin with calmidazolium increases ENaC activity presumably by regulating MARCKS and MLP-1. Activated calmodulin can regulate MARCKS and MLP-1 in two ways. Calmodulin can bind to the effector domain of MARCKS or MLP-1, inactivating both proteins by causing their dissociation from the membrane. Mutations in MARCKS that prevent calmodulin association prevent dissociation of MARCKS from the membrane. Calmodulin also activates CaM kinase II (CaMKII). An inhibitor of CaMKII (KN93) increases ENaC activity, MARCKS association with ENaC, and promotes MARCKS movement to a membrane fraction. CaMKII phosphorylates filamin. Filamin is an essential component of the cytoskeleton and promotes association of ENaC, MARCKS, and MLP-1. Disruption of the cytoskeleton with cytochalasin E reduces ENaC activity. CaMKII phosphorylation of filamin disrupts the cytoskeleton and the association of MARCKS, MLP-1, and ENaC, thereby reducing ENaC open probability. Taken together, these findings suggest calmodulin and CaMKII modulate ENaC activity by destabilizing the association between the actin cytoskeleton, ENaC, and MARCKS, or MLP-1 at the apical membrane.
Keywords: CaMKII; ENaC; MARCKS; calcium; filamin.
Copyright © 2015 the American Physiological Society.
Figures
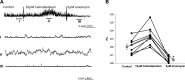
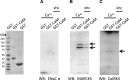


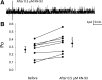




References
-
- Alli AA, Gower WR Jr. The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol 297: C1157–C1167, 2009. - PubMed
-
- Alli AA, Gower WR Jr. Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J Cell Biochem 110: 985–994, 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

