Different toxic effects of YTX in tumor K-562 and lymphoblastoid cell lines
- PMID: 26136685
- PMCID: PMC4469822
- DOI: 10.3389/fphar.2015.00124
Different toxic effects of YTX in tumor K-562 and lymphoblastoid cell lines
Abstract
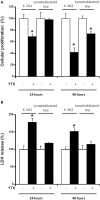
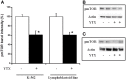
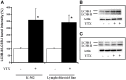
Yessotoxin (YTX) modulates cellular phosphodiesterases (PDEs). In this regard, opposite effects had been described in the tumor model K-562 cell line and fresh human lymphocytes in terms of cell viability, cyclic adenosine 3',5'-cyclic monophosphate (cAMP) production and protein expression after YTX treatment. Studies in depth of the pathways activated by YTX in K-562 cell line, have demonstrated the activation of two different cell death types, apoptosis, and autophagy after 24 and 48 h of treatment, respectively. Furthermore, the key role of type 4A PDE (PDE4A) in both pathways activated by YTX was demonstrated. Therefore, taking into account the differences between cellular lines and fresh cells, a study of cell death pathways activated by YTX in a non-tumor cell line with mitotic activity, was performed. The cellular model used was the lymphoblastoid cell line that represents a non-tumor model with normal apoptotic and mitotic machinery. In this context, cell viability and cell proliferation, expression of proteins involved in cell death activated by YTX and mitochondrial mass, were studied after the incubation with the toxin. Opposite to the tumor model, no cell death activation was observed in lymphoblastoid cell line in the presence of YTX. In this sense, variations in apoptosis hallmarks were not detected in the lymphoblastoid cell line after YTX incubation, whereas this type I of programmed cell death was observed in K-562 cells. On the other hand, autophagy cell death was triggered in this cellular line, while other autophagic process is suggested in lymphoblastoid cells. These YTX effects are related to PDE4A in both cellular lines. In addition, while cell death is triggered in K-562 cells after YTX treatment, in lymphoblastoid cells the toxin stops cellular proliferation. These results point to YTX as a specific toxic compound of tumor cells, since in the non-tumor lymphoblastoid cell line, no cell death hallmarks are observed.
Keywords: K-562; Yessotoxin; apoptosis; autophagy; lymphoblastoid line.
Figures





Similar articles
-
Yessotoxin, a Promising Therapeutic Tool.Mar Drugs. 2016 Jan 28;14(2):30. doi: 10.3390/md14020030. Mar Drugs. 2016. PMID: 26828502 Free PMC article. Review.
-
Key role of phosphodiesterase 4A (PDE4A) in autophagy triggered by yessotoxin.Toxicology. 2015 Mar 2;329:60-72. doi: 10.1016/j.tox.2015.01.004. Epub 2015 Jan 7. Toxicology. 2015. PMID: 25576684
-
Role of yessotoxin in calcium and cAMP-crosstalks in primary and K-562 human lymphocytes: the effect is mediated by anchor kinase A mitochondrial proteins.J Cell Biochem. 2012 Dec;113(12):3752-61. doi: 10.1002/jcb.24249. J Cell Biochem. 2012. PMID: 22807343
-
Role of AKAP 149-PKA-PDE4A complex in cell survival and cell differentiation processes.Int J Biochem Cell Biol. 2014 Aug;53:89-101. doi: 10.1016/j.biocel.2014.04.028. Epub 2014 May 9. Int J Biochem Cell Biol. 2014. PMID: 24813785
-
Yessotoxin as an apoptotic inducer.Toxicon. 2011 Jun;57(7-8):947-58. doi: 10.1016/j.toxicon.2011.03.012. Epub 2011 Mar 21. Toxicon. 2011. PMID: 21426912 Review.
Cited by
-
MTMR3 is upregulated in patients with breast cancer and regulates proliferation, cell cycle progression and autophagy in breast cancer cells.Oncol Rep. 2019 Nov;42(5):1915-1923. doi: 10.3892/or.2019.7292. Epub 2019 Aug 23. Oncol Rep. 2019. PMID: 31485632 Free PMC article.
-
Current Trends and New Challenges in Marine Phycotoxins.Mar Drugs. 2022 Mar 8;20(3):198. doi: 10.3390/md20030198. Mar Drugs. 2022. PMID: 35323497 Free PMC article. Review.
-
Yessotoxin, a Marine Toxin, Exhibits Anti-Allergic and Anti-Tumoural Activities Inhibiting Melanoma Tumour Growth in a Preclinical Model.PLoS One. 2016 Dec 14;11(12):e0167572. doi: 10.1371/journal.pone.0167572. eCollection 2016. PLoS One. 2016. PMID: 27973568 Free PMC article.
-
Yessotoxin, a Promising Therapeutic Tool.Mar Drugs. 2016 Jan 28;14(2):30. doi: 10.3390/md14020030. Mar Drugs. 2016. PMID: 26828502 Free PMC article. Review.
References
-
- Bae-Jump V. L., Zhou C., Boggess J. F., Whang Y. E., Barroilhet L., Gehrig P. A. (2010). Rapamycin inhibits cell proliferation in type I and type II endometrial carcinomas: a search for biomarkers of sensitivity to treatment. Gynecol. Oncol. 119, 579–585. 10.1016/j.ygyno.2010.08.025 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

