Cidan inhibits liver cancer cell growth by reducing COX-2 and VEGF expression and cell cycle arrest
- PMID: 26136881
- PMCID: PMC4471759
- DOI: 10.3892/etm.2015.2351
Cidan inhibits liver cancer cell growth by reducing COX-2 and VEGF expression and cell cycle arrest
Erratum in
-
CORRIGENDUM.Exp Ther Med. 2015 Oct;10(4):1609. doi: 10.3892/etm.2015.2655. Epub 2015 Jul 24. Exp Ther Med. 2015. PMID: 26622534 Free PMC article.
Abstract
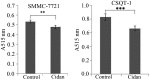
Cidan is a traditional Chinese medicine formula that has been used for >10 years as an antitumor drug. In the present study, the antitumor effect of cidan on hepatocellular carcinoma (HCC) and the underlying molecular mechanisms were investigated. A total of 372 patients with primary HCC, as confirmed by pathological examination in the Eastern Hepatobiliary Surgery Hospital and Beijing Oncology Hospital of Weida TCM, were prospectively enrolled in the study. In total, 92 patients were treated with cidan capsules for three months postoperatively, while 280 patients served as controls. The efficacy of cidan was analyzed by monitoring associated symptoms and liver function tests, including measuring the levels of α-1-fetoprotein, α-L-fucosidase, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. In addition, in vivo analysis was performed using mice Hepa1-6 xenograft models, while in vitro studies were performed with SMMC-7721 and CSQT-1 cells; this included cidan-dependent cell viability and migration assays, cell cycle analyses and the evaluation of cidan effects on cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) mRNA transcription rates using quantitative polymerase chain reaction. The postoperative two-year overall survival (77 and 58% for the cidan and control groups, respectively; P<0.01) and disease-free survival (36 and 24% for the cidan and control groups, respectively; P<0.01) rates were superior in the cidan-treated group when compared with the control. In addition, the size and weight of the tumor xenografts in the C57BL/6 mice were significantly reduced in a time- and dose-dependent manner following cidan treatment (P<0.01). Cidan significantly reduced the cell viability of SMMC-7721 and CSQT-1 cells after four and five days when compared with the control (P<0.01). Furthermore, COX-2 and VEGF mRNA expression levels decreased following cidan treatment (P<0.01), and cidan treatment resulted in enhanced G1 and G2/M cell cycle arrest of CSQT-1 cells. Therefore, cidan effectively inhibited cell proliferation, reduced cell viability and downregulated COX-2 and VEGF expression levels in hepatoma cells.
Keywords: Rhizoma Curcumae; cidan; cyclooxygenase-2; primary hepatocellular carcinoma; vascular endothelial growth factor.
Figures





References
-
- Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta-analysis. Hemodial Int. 2013;17:532–541. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
