Non-invasive measurement of choroidal volume change and ocular rigidity through automated segmentation of high-speed OCT imaging
- PMID: 26137373
- PMCID: PMC4467714
- DOI: 10.1364/BOE.6.001694
Non-invasive measurement of choroidal volume change and ocular rigidity through automated segmentation of high-speed OCT imaging
Abstract
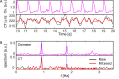
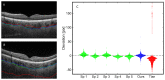
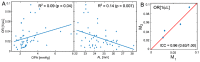
We have developed a novel optical approach to determine pulsatile ocular volume changes using automated segmentation of the choroid, which, together with Dynamic Contour Tonometry (DCT) measurements of intraocular pressure (IOP), allows estimation of the ocular rigidity (OR) coefficient. Spectral Domain Optical Coherence Tomography (OCT) videos were acquired with Enhanced Depth Imaging (EDI) at 7Hz during ~50 seconds at the fundus. A novel segmentation algorithm based on graph search with an edge-probability weighting scheme was developed to measure choroidal thickness (CT) at each frame. Global ocular volume fluctuations were derived from frame-to-frame CT variations using an approximate eye model. Immediately after imaging, IOP and ocular pulse amplitude (OPA) were measured using DCT. OR was calculated from these peak pressure and volume changes. Our automated segmentation algorithm provides the first non-invasive method for determining ocular volume change due to pulsatile choroidal filling, and the estimation of the OR constant. Future applications of this method offer an important avenue to understanding the biomechanical basis of ocular pathophysiology.
Keywords: (170.3880) Medical and biological imaging; (170.4460) Ophthalmic optics and devices; (170.6935) Tissue characterization.
Figures


References
-
- Burgoyne C. F., Downs J. C., Bellezza A. J., Suh J. K., Hart R. T., “The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage,” Prog. Retin. Eye Res. 24(1), 39–73 (2005).10.1016/j.preteyeres.2004.06.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
