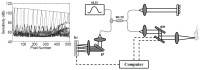
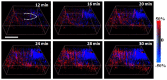
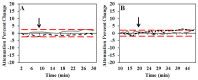
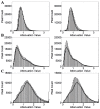
Localization of cortical tissue optical changes during seizure activity in vivo with optical coherence tomography
- PMID: 26137382
- PMCID: PMC4467710
- DOI: 10.1364/BOE.6.001812
Localization of cortical tissue optical changes during seizure activity in vivo with optical coherence tomography
Abstract
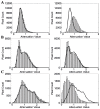
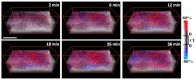
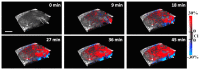
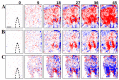
Optical coherence tomography (OCT) is a high resolution, minimally invasive imaging technique, which can produce depth-resolved cross-sectional images. In this study, OCT was used to detect changes in the optical properties of cortical tissue in vivo in mice during the induction of global (pentylenetetrazol) and focal (4-aminopyridine) seizures. Through the use of a confidence interval statistical method on depth-resolved volumes of attenuation coefficient, we demonstrated localization of regions exhibiting both significant positive and negative changes in attenuation coefficient, as well as differentiating between global and focal seizure propagation.
Keywords: (100.2960) Image analysis; (110.4500) Optical coherence tomography; (170.3880) Medical and biological imaging.
Figures