Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: A randomized, placebo-controlled, phase 1 trial
- PMID: 26137397
- PMCID: PMC4485742
- DOI: 10.1080/2162402X.2014.1001217
Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: A randomized, placebo-controlled, phase 1 trial
Abstract
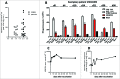
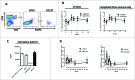
VEGFR-2 is expressed on tumor vasculature and a target for anti-angiogenic intervention. VXM01 is a first in kind orally applied tumor vaccine based on live, attenuated Salmonella bacteria carrying an expression plasmid, encoding VEGFR-2. We here studied the safety, tolerability, T effector (Teff), T regulatory (Treg) and humoral responses to VEGFR2 and anti-angiogenic effects in advanced pancreatic cancer patients in a randomized, dose escalation phase I clinical trial. Results of the first 3 mo observation period are reported. Locally advanced or metastatic, pancreatic cancer patients were enrolled. In five escalating dose groups, 30 patients received VXM01 and 15 placebo on days 1, 3, 5, and 7. Treatment was well tolerated at all dose levels. No dose-limiting toxicities were observed. Salmonella excretion and salmonella-specific humoral immune responses occurred in the two highest dose groups. VEGFR2 specific Teff, but not Treg responses were overall increased in vaccinated patients. We furthermore observed a significant reduction of tumor perfusion after 38 d in vaccinated patients together with increased levels of serum biomarkers indicative of anti-angiogenic activity, VEGF-A, and collagen IV. Vaccine specific Teff responses significantly correlated with reductions of tumor perfusion and high levels of preexisting VEGFR2-specific Teff while those showing no antiangiogenic activity had low levels of preexisting VEGFR2 specific Teff, showed a transient early increase of VEGFR2-specific Treg and reduced levels of VEGFR2-specific Teff at later time points - pointing to the possibility that early anti-angiogenic activity might be based at least in part on specific reactivation of preexisting memory T cells.
Keywords: VEGFR2; anti-angiogenic treatment; cancer immunotherapy; oral vaccination; pancreatic cancer.
Figures




References
-
- Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev 2008; 222:117-28; PMID:; http://dx.doi.org/10.1111/j.1600-065X.2008.00613.x - DOI - PubMed
-
- Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine 2007; 25:7848-57; PMID:; http://dx.doi.org/10.1016/j.vaccine.2007.08.027 - DOI - PubMed
-
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013; 153:13-9; PMID:; http://dx.doi.org/10.1093/jb/mvs136 - DOI - PMC - PubMed
-
- Bruce D, Tan PH. Vascular endothelial growth factor receptors and the therapeutic targeting of angiogenesis in cancer: where do we go from here? Cell Commun Adhes 2011; 18:85-103; PMID:; http://dx.doi.org/10.3109/15419061.2011.619673 - DOI - PubMed
-
- Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 2012; 12:699-709; PMID:; http://dx.doi.org/10.1038/nrc3366 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
