Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody
- PMID: 26137406
- PMCID: PMC4485828
- DOI: 10.4161/2162402X.2014.989776
Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody
Abstract
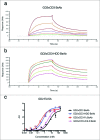
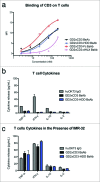
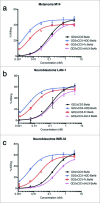
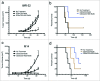
Bispecific antibodies (BsAbs) have proven highly efficient T cell recruiters for cancer immunotherapy by virtue of one tumor antigen-reactive single chain variable fragment (scFv) and another that binds CD3. In order to enhance the antitumor potency of these tandem scFv BsAbs (tsc-BsAbs), we exploited the dimerization domain of the human transcription factor HNF1α to enhance the avidity of a tsc-BsAb to the tumor antigen disialoganglioside GD2 while maintaining functional monovalency to CD3 to limit potential toxicity. The dimeric tsc-BsAb showed increased avidity to GD2, enhanced T cell mediated killing of neuroblastoma and melanoma cell lines in vitro (32-37 fold), exhibited a near 4-fold improvement in serum half-life, and enhanced tumor ablation in mouse xenograft models. We propose that the use of this HNF1α-derived dimerization tag may be a novel and effective strategy to increase the potency of T-cell engaging antibodies for clinical cancer immunotherapy.
Keywords: BiTE; BiTE, bispecific T-cell engager; Bispecific antibody; BsAb, bispecific antibody; GD2; HDD, HNF1α dimerization domain; T-cell; ganglioside; immunotherapy; melanoma; neuroblastoma; tsc-BsAb, tandem single-chain bispecific antibody.
Figures

References
-
- Choi BD, Cai M, Bigner DD, Mehta AI, Kuan CT, Sampson JH. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin Biol Ther 2011; 11:843-53; PMID:; http://dx.doi.org/10.1517/14712598.2011.572874 - DOI - PubMed
-
- Dreier T, Baeuerle PA, Fichtner I, Grün M, Schlereth B, Lorenczewski G, Kufer P, Lutterbüse R, Riethmüller G, Gjorstrup P, et al.. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol 2003; 170:4397-402; PMID:; http://dx.doi.org/10.4049/jimmunol.170.8.4397 - DOI - PubMed
-
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et al.. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321:974-7; PMID:; http://dx.doi.org/10.1126/science.1158545 - DOI - PubMed
-
- Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al.. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493-8; PMID:; http://dx.doi.org/10.1200/JCO.2010.32.7270 - DOI - PubMed
-
- Torisu-Itakura H, Schoellhammer HF, Sim MS, Irie RF, Hausmann S, Raum T, Baeuerle PA, Morton DL. Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific BiTE antibody that engages patient-derived T cells. J Immunother 2011; 34:597-605; PMID:; http://dx.doi.org/10.1097/CJI.0b013e3182307fd8 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
