Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs
- PMID: 26137500
- PMCID: PMC4484766
- DOI: 10.1016/j.gendis.2015.02.001
Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs
Abstract
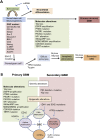
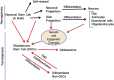
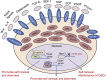
Cancer stem cells (CSCs) or cancer initiating cells (CICs) maintain self-renewal and multilineage differentiation properties of various tumors, as well as the cellular heterogeneity consisting of several subpopulations within tumors. CSCs display the malignant phenotype, self-renewal ability, altered genomic stability, specific epigenetic signature, and most of the time can be phenotyped by cell surface markers (e.g., CD133, CD24, and CD44). Numerous studies support the concept that non-stem cancer cells (non-CSCs) are sensitive to cancer therapy while CSCs are relatively resistant to treatment. In glioblastoma stem cells (GSCs), there is clonal heterogeneity at the genetic level with distinct tumorigenic potential, and defined GSC marker expression resulting from clonal evolution which is likely to influence disease progression and response to treatment. Another level of complexity in glioblastoma multiforme (GBM) tumors is the dynamic equilibrium between GSCs and differentiated non-GSCs, and the potential for non-GSCs to revert (dedifferentiate) to GSCs due to epigenetic alteration which confers phenotypic plasticity to the tumor cell population. Moreover, exposure of the differentiated GBM cells to therapeutic doses of temozolomide (TMZ) or ionizing radiation (IR) increases the GSC pool both in vitro and in vivo. This review describes various subtypes of GBM, discusses the evolution of CSC models and epigenetic plasticity, as well as interconversion between GSCs and differentiated non-GSCs, and offers strategies to potentially eliminate GSCs.
Keywords: Cancer stem cells; Dedifferentiation; Epigenetic; GBM plasticity; GBM stem cells; Glioblastoma; Stemness.
Figures

References
-
- Jemal A., Murray T., Ward E. Cancer statistics. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Hegi M.E., Diserens A.C., Godard S. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10:1871–1874. - PubMed
-
- Hegi M.E., Diserens A.C., Gorlia T. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. - PubMed
-
- Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
