In Vivo Application of Tissue-Engineered Veins Using Autologous Peripheral Whole Blood: A Proof of Concept Study
- PMID: 26137509
- PMCID: PMC4457407
- DOI: 10.1016/j.ebiom.2014.09.001
In Vivo Application of Tissue-Engineered Veins Using Autologous Peripheral Whole Blood: A Proof of Concept Study
Abstract
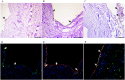
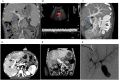
Vascular diseases are increasing health problems affecting > 25 million individuals in westernized societies. Such patients could benefit from transplantation of tissue-engineered vascular grafts using autologous cells. One challenge that has limited this development is the need for cell isolation, and risks associated with ex vivo expanded stem cells. Here we demonstrate a novel approach to generate transplantable vascular grafts using decellularized allogeneic vascular scaffolds, repopulated with peripheral whole blood (PWB) in vitro in a bioreactor. Circulating, VEGFR-2 +/CD45 + and a smaller fraction of VEGFR-2 +/CD14 + cells contributed to repopulation of the graft. SEM micrographs showed flat cells on the luminal surface of the grafts consistent with endothelial cells. For clinical validation, two autologous PWB tissue-engineered vein conduits were prepared and successfully used for by-pass procedures in two pediatric patients. These results provide a proof of principle for the generation of transplantable vascular grafts using a simple autologous blood sample, making it clinically feasible globally.
Keywords: Endothelial precursors; Tissue-engineering; Vascular diseases; Vein conduits.
Figures



References
-
- Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. - PubMed
-
- Deutsch M., Meinhart J., Zilla P. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J. Vasc. Surg. 2009;49(2):352–362. (discussion 62) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

