Mitochondrial-dependent Autoimmunity in Membranous Nephropathy of IgG4-related Disease
- PMID: 26137589
- PMCID: PMC4485911
- DOI: 10.1016/j.ebiom.2015.03.003
Mitochondrial-dependent Autoimmunity in Membranous Nephropathy of IgG4-related Disease
Abstract
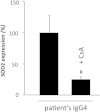
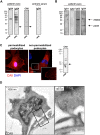
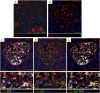
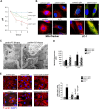
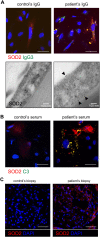
The pathophysiology of glomerular lesions of membranous nephropathy (MN), including seldom-reported IgG4-related disease, is still elusive. Unlike in idiopathic MN where IgG4 prevails, in this patient IgG3 was predominant in glomerular deposits in the absence of circulating anti-phospholipase A2 receptor antibodies, suggesting a distinct pathologic process. Here we documented that IgG4 retrieved from the serum of our propositus reacted against carbonic anhydrase II (CAII) at the podocyte surface. In patient's biopsy, glomerular CAII staining increased and co-localized with subepithelial IgG4 deposits along the capillary walls. Patient's IgG4 caused a drop in cell pH followed by mitochondrial dysfunction, excessive ROS production and cytoskeletal reorganization in cultured podocytes. These events promoted mitochondrial superoxide-dismutase-2 (SOD2) externalization on the plasma membrane, becoming recognizable by complement-binding IgG3 anti-SOD2. Among patients with IgG4-related disease only sera of those with IgG4 anti-CAII antibodies caused low intracellular pH and mitochondrial alterations underlying SOD2 externalization. Circulating IgG4 anti-CAII can cause podocyte injury through processes of intracellular acidification, mitochondrial oxidative stress and neoantigen induction in patients with IgG4 related disease. The onset of MN in a subset of patients could be due to IgG4 antibodies recognizing CAII with consequent exposure of mitochondrial neoantigen in the context of multifactorial pathogenesis of disease.
Keywords: Carbonic anhydrase II; IgG4-related disease; Membranous nephropathy; Podocyte; Superoxide dismutase 2.
Figures







Comment in
-
Membranous Nephropathy: The Journey Continues ….EBioMedicine. 2015 Mar 18;2(5):374-5. doi: 10.1016/j.ebiom.2015.03.013. eCollection 2015 May. EBioMedicine. 2015. PMID: 26137581 Free PMC article. No abstract available.
References
-
- Stone J.H., Zen Y., Deshpande V. IgG4-related disease. N. Engl. J. Med. 2012;366:539–551. - PubMed
-
- Saeki T., Nishi S., Imai N. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–1023. - PubMed
-
- Alexander M.P., Larsen C.P., Gibson I.W. Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int. 2013;83:455–462. - PubMed
-
- Cravedi P., Abbate M., Gagliardini E. Membranous nephropathy associated with IgG4-related disease. Am. J. Kidney Dis. 2011;58:272–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

