Surveillance of adverse events following immunization with meningococcal group C conjugate vaccine: Tuscany, 2005-2012
- PMID: 26137788
- PMCID: PMC4718312
Surveillance of adverse events following immunization with meningococcal group C conjugate vaccine: Tuscany, 2005-2012
Abstract
Introduction: Post-licensure vaccine safety studies are essential to identify uncommon events that may be difficult to assess during pre-licensure studies. The aim of our study was to evaluate the safety of serogroup C meningococcal conjugate (MCC) vaccine in Tuscany from 2005 to 2012.
Methods: All adverse events (AEs) to MCC vaccine notified from 2005 to 2012 were obtained from the regional health authority.
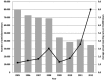
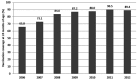
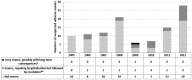
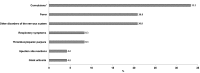
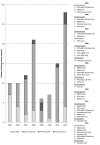
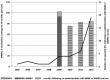
Results: Following 451,570 doses administered, 110 suspected AEs were notified (mean annual reporting rate: 2.8/10,000 doses). The most frequently AE reported was fever (60%), followed by swelling at the injection site (11%) and febrile seizures (10%). Overall, 77.3% of cases were not severe, while 21.8% required hospitalization. Almost four months after the receipt of the vaccine, a one-year-old infant was diagnosed with a pervasive developmental disorder with disturbance of speech, but any link with the vaccinations received was refuted. Most AEs (80.9%) occurred after co-administration with other vaccines, especially with MMR or MMRV vaccines (42.7%) or the DTPa-HBV-IPV/Hib vaccine (33.7%).
Discussion: Our study confirmed the high level of safety of MCC vaccine in Tuscany: AEs proved rare and all cases had only temporary and self-resolving consequences. As usually only the most severe suspected AEs are reported, the true proportion of AEs requiring hospitalization was most probably overestimated, and it is plausible that most of these cases were in fact only temporally related.
Figures
Similar articles
-
Simultaneous administration of meningococcal C conjugate vaccine and diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine in children: a randomized double-blind study.Clin Invest Med. 2002 Dec;25(6):243-51. Clin Invest Med. 2002. PMID: 12516995 Clinical Trial.
-
A combined Haemophilus influenzae type B Neisseria meningitidis serogroup C tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when coadministered with diphtheria, tetanus, acellular pertussis hepatitis B-inactivated poliovirus at 3, 5 and 11 months of age: results of an open, randomized, controlled study.Pediatr Infect Dis J. 2013 May;32(5):521-9. doi: 10.1097/INF.0b013e31827e22e3. Pediatr Infect Dis J. 2013. PMID: 23190785 Clinical Trial.
-
Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the ACTION follow-up project.Vaccine. 2014 Feb 7;32(7):793-9. doi: 10.1016/j.vaccine.2013.12.044. Epub 2014 Jan 5. Vaccine. 2014. PMID: 24397902
-
Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines.Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S109-18. doi: 10.1097/INF.0b013e318199f62d. Pediatr Infect Dis J. 2009. PMID: 19325447 Review.
-
New combination vaccines: DTaP-IPV (Kinrix) and DTaP-IPV/Hib (Pentacel).Ann Pharmacother. 2010 Mar;44(3):515-23. doi: 10.1345/aph.1M468. Ann Pharmacother. 2010. PMID: 20197476 Review.
Cited by
-
[Analysis of the Vaccine Adverse Event Reporting System in Brazil, 2014 to 2016Análisis del Sistema de Información de Vigilancia de Eventos Adversos Posvacunación en Brasil, 2014 a 2016].Rev Panam Salud Publica. 2018 Feb 28;42:e12. doi: 10.26633/RPSP.2018.12. eCollection 2018. Rev Panam Salud Publica. 2018. PMID: 31093041 Free PMC article. Portuguese.
-
Co-administration of vaccines: a focus on tetravalent Measles-Mumps-Rubella-Varicella (MMRV) and meningococcal C conjugate vaccines.Hum Vaccin Immunother. 2020 Jun 2;16(6):1313-1321. doi: 10.1080/21645515.2019.1688032. Epub 2019 Dec 10. Hum Vaccin Immunother. 2020. PMID: 31810408 Free PMC article.
-
Safety of routine childhood vaccine coadministration versus separate vaccination.BMJ Glob Health. 2022 Sep;7(9):e008215. doi: 10.1136/bmjgh-2021-008215. BMJ Glob Health. 2022. PMID: 36162867 Free PMC article.
References
-
- Wilder-Smith A. Meningococcal vaccine in travelers. Curr Opin Infect Dis. 2007;20:454–460. - PubMed
-
- European Centre for Disease Prevention and Control , author. Surveillance of invasive bacterial diseases in Europe, 2011. Stockholm: 2013.
-
- World Health Organization , author. Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec. 2011;47:521–540. - PubMed
-
- Bonanni P, Bechini A, Boccalini S, et al. Igiene e Sanità Pubblica. 2012. Epidemiologia e prevenzione delle malattie infettive; pp. 355–447.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
