Riboswitch-mediated Attenuation of Transgene Cytotoxicity Increases Adeno-associated Virus Vector Yields in HEK-293 Cells
- PMID: 26137851
- PMCID: PMC4817922
- DOI: 10.1038/mt.2015.123
Riboswitch-mediated Attenuation of Transgene Cytotoxicity Increases Adeno-associated Virus Vector Yields in HEK-293 Cells
Abstract
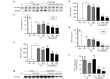
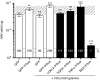
Cytotoxicity of transgenes carried by adeno-associated virus (AAV) vectors might be desired, for instance, in oncolytic virotherapy or occur unexpectedly in exploratory research when studying sparsely characterized genes. To date, most AAV-based studies use constitutively active promoters (e.g., the CMV promoter) to drive transgene expression, which often hampers efficient AAV production due to cytotoxic, antiproliferative, or unknown transgene effects interfering with producer cell performance. Therefore, we explored artificial riboswitches as novel tools to control transgene expression during AAV production in mammalian cells. Our results demonstrate that the guanine-responsive GuaM8HDV aptazyme efficiently attenuates transgene expression and associated detrimental effects, thereby boosting AAV vector yields up to 23-fold after a single addition of guanine. Importantly, riboswitch-harboring vectors preserved their ability to express functional transgene at high levels in the absence of ligand, as demonstrated in a mouse model of AAV-TGFβ1-induced pulmonary fibrosis. Thus, our study provides the first application-ready biotechnological system-based on aptazymes, which should enable high viral vector yields largely independent of the transgene used. Moreover, the RNA-intrinsic, small-molecule regulatable mode of action of riboswitches provides key advantages over conventional transcription factor-based regulatory systems. Therefore, such riboswitch vectors might be ultimately applied to temporally control therapeutic transgene expression in vivo.
Figures



Similar articles
-
Synthetic riboswitches for external regulation of genes transferred by replication-deficient and oncolytic adenoviruses.Nucleic Acids Res. 2012 Nov;40(21):e167. doi: 10.1093/nar/gks734. Epub 2012 Aug 9. Nucleic Acids Res. 2012. PMID: 22885302 Free PMC article.
-
Reversible Gene Regulation in Mammalian Cells Using Riboswitch-Engineered Vesicular Stomatitis Virus Vector.ACS Synth Biol. 2019 Sep 20;8(9):1976-1982. doi: 10.1021/acssynbio.9b00177. Epub 2019 Aug 15. ACS Synth Biol. 2019. PMID: 31415142
-
Adeno-Associated Virus Serotype-Specific Inverted Terminal Repeat Sequence Role in Vector Transgene Expression.Hum Gene Ther. 2020 Feb;31(3-4):151-162. doi: 10.1089/hum.2019.274. Hum Gene Ther. 2020. PMID: 31914802 Free PMC article.
-
Riboswitches for Controlled Expression of Therapeutic Transgenes Delivered by Adeno-Associated Viral Vectors.Pharmaceuticals (Basel). 2021 Jun 10;14(6):554. doi: 10.3390/ph14060554. Pharmaceuticals (Basel). 2021. PMID: 34200913 Free PMC article. Review.
-
Adeno-associated virus vectors: activity and applications in the CNS.J Neurosci Methods. 2000 Jun 1;98(2):95-104. doi: 10.1016/s0165-0270(00)00183-7. J Neurosci Methods. 2000. PMID: 10880823 Review.
Cited by
-
Suppression of toxic transgene expression by optimized artificial miRNAs increases AAV vector yields in HEK-293 cells.Mol Ther Methods Clin Dev. 2024 Jun 10;32(3):101280. doi: 10.1016/j.omtm.2024.101280. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39015407 Free PMC article.
-
Synthetic switch-based baculovirus for transgene expression control and selective killing of hepatocellular carcinoma cells.Nucleic Acids Res. 2018 Sep 6;46(15):e93. doi: 10.1093/nar/gky447. Nucleic Acids Res. 2018. PMID: 29905834 Free PMC article.
-
Insights from the Construction of Adenovirus-Based Vaccine Candidates against SARS-CoV-2: Expecting the Unexpected.Viruses. 2023 Oct 25;15(11):2155. doi: 10.3390/v15112155. Viruses. 2023. PMID: 38005833 Free PMC article.
-
Engineering of Small Ribozymes Acting on RNA: What is Needed to Make a New Function Work with an Existing Catalyst?Chembiochem. 2025 May 27;26(10):e202500213. doi: 10.1002/cbic.202500213. Epub 2025 May 21. Chembiochem. 2025. PMID: 40295187 Free PMC article. Review.
-
The role of small molecules in cell and gene therapy.RSC Med Chem. 2020 Dec 24;12(3):330-352. doi: 10.1039/d0md00221f. eCollection 2021 Mar 1. RSC Med Chem. 2020. PMID: 34046619 Free PMC article. Review.
References
-
- Grieger, JC and Samulski, RJ (2012). Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol 507: 229–254. - PubMed
-
- Mingozzi, F and High, KA (2011). Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 12: 341–355. - PubMed
-
- Roche-Molina, M, Sanz-Rosa, D, Cruz, FM, García-Prieto, J, López, S, Abia, R et al. (2015). Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arterioscler Thromb Vasc Biol 35: 50–59. - PubMed
-
- Werfel, S, Jungmann, A, Lehmann, L, Ksienzyk, J, Bekeredjian, R, Kaya, Z et al. (2014). Rapid and highly efficient inducible cardiac gene knockout in adult mice using AAV-mediated expression of Cre recombinase. Cardiovasc Res 104: 15–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

