Asymmetric synthesis of N-allylic indoles via regio- and enantioselective allylation of aryl hydrazines
- PMID: 26137886
- PMCID: PMC4506507
- DOI: 10.1038/ncomms8616
Asymmetric synthesis of N-allylic indoles via regio- and enantioselective allylation of aryl hydrazines
Abstract
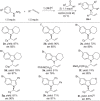
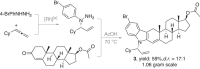
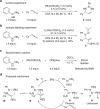
The asymmetric synthesis of N-allylic indoles is important for natural product synthesis and pharmaceutical research. The regio- and enantioselective N-allylation of indoles is a true challenge due to the favourable C3-allylation. We develop here a new strategy to the asymmetric synthesis of N-allylic indoles via rhodium-catalysed N-selective coupling of aryl hydrazines with allenes followed by Fischer indolization. The exclusive N-selectivities and good to excellent enantioselectivities are achieved applying a rhodium(I)/DTBM-Segphos or rhodium(I)/DTBM-Binap catalyst. This method permits the practical synthesis of valuable chiral N-allylated indoles, and avoids the N- or C-selectivity issue.
Figures


References
-
- Pasquali G., Porto D. D. & Fett-Neto A. G. Metabolic engineering of cell cultures versus whole plant complexity in production of bioactive monoterpene indole alkaloids: recent progress related to old dilemma. J. Biosci. Bioeng. 101, 287–296 (2006). - PubMed
-
- Lewis S. E. Recent advances in the chemistry of macroline, sarpagine and ajmaline-related indole alkaloids. Tetrahedron 62, 8655–8681 (2006).
-
- O'Connor S. E. & Maresh J. J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 23, 532–547 (2006). - PubMed
-
- Higuichi K. & Kawasaki T. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 24, 843–868 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

