Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys
- PMID: 26138104
- PMCID: PMC4653134
- DOI: 10.1126/science.aab3886
Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys
Abstract
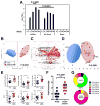
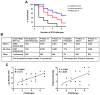
Preclinical studies of viral vector-based HIV-1 vaccine candidates have previously shown partial protection against neutralization-resistant virus challenges in rhesus monkeys. In this study, we evaluated the protective efficacy of adenovirus serotype 26 (Ad26) vector priming followed by purified envelope (Env) glycoprotein boosting. Rhesus monkeys primed with Ad26 vectors expressing SIVsmE543 Env, Gag, and Pol and boosted with AS01B-adjuvanted SIVmac32H Env gp140 demonstrated complete protection in 50% of vaccinated animals against a series of repeated, heterologous, intrarectal SIVmac251 challenges that infected all controls. Protective efficacy correlated with the functionality of Env-specific antibody responses. Comparable protection was also observed with a similar Ad/Env vaccine against repeated, heterologous, intrarectal SHIV-SF162P3 challenges. These data demonstrate robust protection by Ad/Env vaccines against acquisition of neutralization-resistant virus challenges in rhesus monkeys.
Copyright © 2015, American Association for the Advancement of Science.
Figures



References
-
- Fauci AS, Marston HD. Ending AIDS--is an HIV vaccine necessary? N Engl J Med. 2014 Feb 6;370:495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI078526/AI/NIAID NIH HHS/United States
- AI095985/AI/NIAID NIH HHS/United States
- R01 AI102691/AI/NIAID NIH HHS/United States
- AI096040/AI/NIAID NIH HHS/United States
- AI080289/AI/NIAID NIH HHS/United States
- R37 AI080289/AI/NIAID NIH HHS/United States
- R01 AI084794/AI/NIAID NIH HHS/United States
- AI078526/AI/NIAID NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- U19 AI096040/AI/NIAID NIH HHS/United States
- R01 AI102660/AI/NIAID NIH HHS/United States
- OD011170/OD/NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- AI084794/AI/NIAID NIH HHS/United States
- U19 AI095985/AI/NIAID NIH HHS/United States
- AI102660/AI/NIAID NIH HHS/United States
- AI102691/AI/NIAID NIH HHS/United States
- R01 OD011170/OD/NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R01 AI080289/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

