Impaired protein translation in Drosophila models for Charcot-Marie-Tooth neuropathy caused by mutant tRNA synthetases
- PMID: 26138142
- PMCID: PMC4506996
- DOI: 10.1038/ncomms8520
Impaired protein translation in Drosophila models for Charcot-Marie-Tooth neuropathy caused by mutant tRNA synthetases
Erratum in
-
Corrigendum: Impaired protein translation in Drosophila models for Charcot-Marie-Tooth neuropathy caused by mutant tRNA synthetases.Nat Commun. 2016 Jan 21;7:10497. doi: 10.1038/ncomms10497. Nat Commun. 2016. PMID: 26792285 Free PMC article. No abstract available.
Abstract
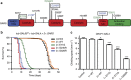
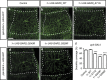
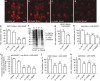
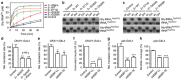
Dominant mutations in five tRNA synthetases cause Charcot-Marie-Tooth (CMT) neuropathy, suggesting that altered aminoacylation function underlies the disease. However, previous studies showed that loss of aminoacylation activity is not required to cause CMT. Here we present a Drosophila model for CMT with mutations in glycyl-tRNA synthetase (GARS). Expression of three CMT-mutant GARS proteins induces defects in motor performance and motor and sensory neuron morphology, and shortens lifespan. Mutant GARS proteins display normal subcellular localization but markedly reduce global protein synthesis in motor and sensory neurons, or when ubiquitously expressed in adults, as revealed by FUNCAT and BONCAT. Translational slowdown is not attributable to altered tRNA(Gly) aminoacylation, and cannot be rescued by Drosophila Gars overexpression, indicating a gain-of-toxic-function mechanism. Expression of CMT-mutant tyrosyl-tRNA synthetase also impairs translation, suggesting a common pathogenic mechanism. Finally, genetic reduction of translation is sufficient to induce CMT-like phenotypes, indicating a causal contribution of translational slowdown to CMT.
Figures


References
-
- Pareyson D. & Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 8, 654–667 (2009). - PubMed
-
- Steffes G. & Storkebaum E. Drosophila as a model for CMT peripheral neuropathy: mutations in tRNA synthetases as an example. in Drosophila melanogaster Models of Motor Neuron Disease eds Cauchi R. J. Nova Biomedical (2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

