Effect of Kidney Function on Drug Kinetics and Dosing in Neonates, Infants, and Children
- PMID: 26138291
- PMCID: PMC4661214
- DOI: 10.1007/s40262-015-0298-7
Effect of Kidney Function on Drug Kinetics and Dosing in Neonates, Infants, and Children
Abstract
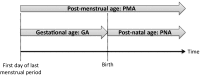
Neonates, infants, and children differ from adults in many aspects, not just in age, weight, and body composition. Growth, maturation and environmental factors affect drug kinetics, response and dosing in pediatric patients. Almost 80% of drugs have not been studied in children, and dosing of these drugs is derived from adult doses by adjusting for body weight/size. As developmental and maturational changes are complex processes, such simplified methods may result in subtherapeutic effects or adverse events. Kidney function is impaired during the first 2 years of life as a result of normal growth and development. Reduced kidney function during childhood has an impact not only on renal clearance but also on absorption, distribution, metabolism and nonrenal clearance of drugs. 'Omics'-based technologies, such as proteomics and metabolomics, can be leveraged to uncover novel markers for kidney function during normal development, acute kidney injury, and chronic diseases. Pharmacometric modeling and simulation can be applied to simplify the design of pediatric investigations, characterize the effects of kidney function on drug exposure and response, and fine-tune dosing in pediatric patients, especially in those with impaired kidney function. One case study of amikacin dosing in neonates with reduced kidney function is presented. Collaborative efforts between clinicians and scientists in academia, industry, and regulatory agencies are required to evaluate new renal biomarkers, collect and share prospective pharmacokinetic, genetic and clinical data, build integrated pharmacometric models for key drugs, optimize and standardize dosing strategies, develop bedside decision tools, and enhance labels of drugs utilized in neonates, infants, and children.
Figures






References
-
- ‘t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children’s hospital. Pediatrics. 2001;108(5):1089–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

