FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans
- PMID: 26138336
- PMCID: PMC4492085
- DOI: 10.1186/s12944-015-0057-7
FTY720 inhibited proinflammatory cytokine release and osteoclastogenesis induced by Aggregatibacter actinomycetemcomitans
Abstract
Background: Periodontitis is a bacteria-driven inflammatory bone loss disease. Previous studies showed that the oral pathogen Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) stimulated the generation of sphingosine 1 phosphate (S1P). In addition, S1P signaling regulated the migration of osteoclast precursors and affected osteoclastogenesis. Furthermore, treatment with FTY720 (also called fingolimod, a modulator of multiple S1P receptors) alleviated osteoporosis and suppressed arthritis in animals. This study determined the effect of FTY720 on proinflammatory cytokine production and osteoclastogenesis in murine bone marrow cells with or without A. actinomycetemcomitans stimulation.
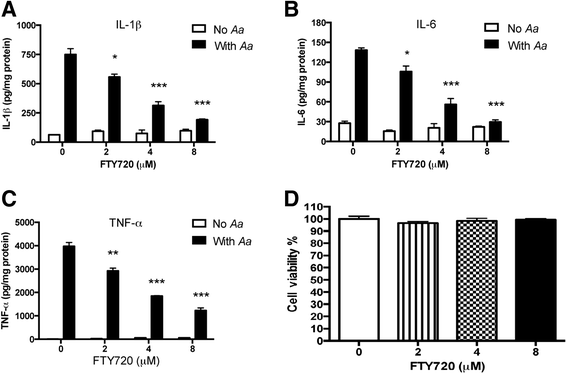
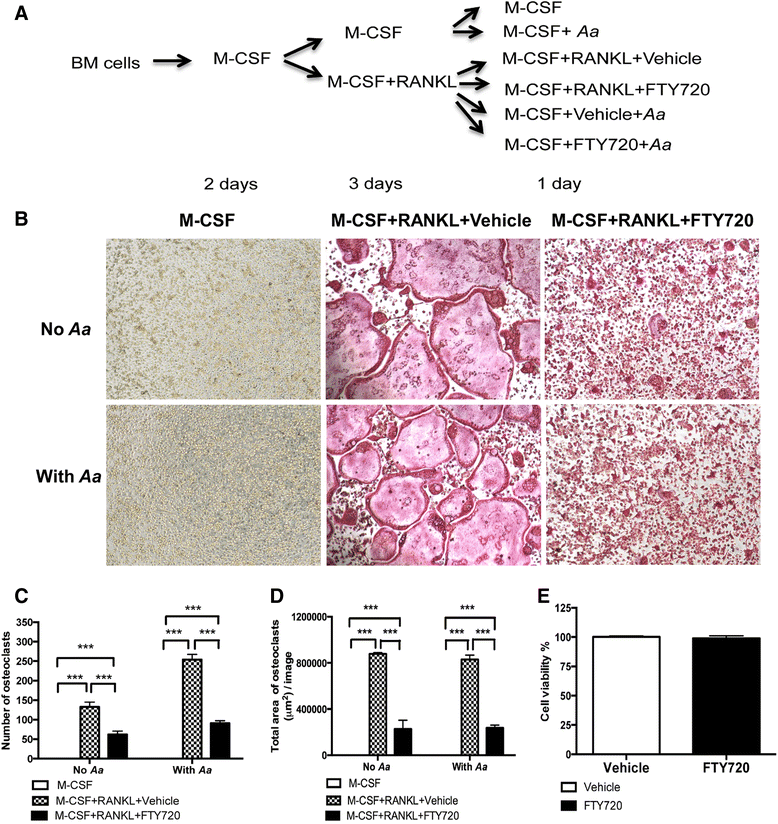
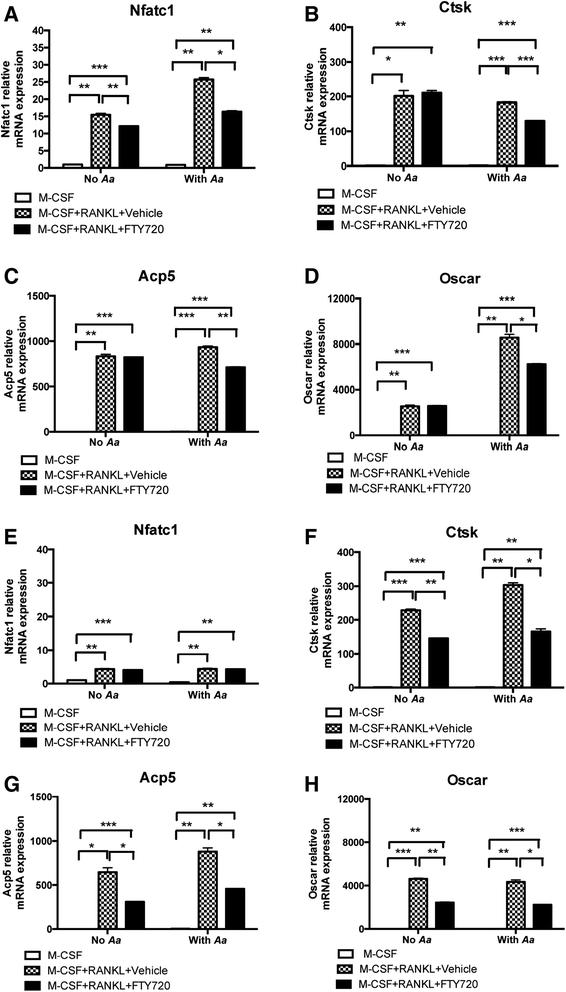
Methods: Murine bone marrow-derived monocytes and macrophages (BMMs) were treated with vehicle ethanol or FTY720, and were either unstimulated or stimulated for 0.5 to 6 h with A. actinomycetemcomitans. The protein levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in the media of BMMs were quantified by enzyme-linked immunosorbent assay (ELISA). Protein expressions, including phosphorylated phosphoinositide 3-kinase (p-PI3K), p-Akt, p-extracellular signal-regulated kinase (p-ERK), PI3K, Akt, and ERK were evaluated by Western blot. In addition, murine bone marrow-derived pre-osteoclasts were treated with macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL) for three days. Then the cells were treated with either vehicle or FTY720 and were either unstimulated or stimulated with A. actinomycetemcomitans for 4 to 24 h. Control cells were treated with M-CSF alone with or without bacterial stimulation. Osteoclasts were stained by tartrate-resistant acid phosphatase (TRAP) staining. The mRNA levels of osteoclastogenic factors, including nuclear factor of activated T-cells cytoplasmic calcineurin-dependent 1 (Nfatc1), cathepsin K (Ctsk), acid phosphatase 5 (Acp5), osteoclast-associated receptor (Oscar), and RANKL were quantified by quantitative real-time polymerase chain reaction (PCR).
Results: FTY720 dose-dependently inhibited IL-1β, IL-6, and TNF-α protein levels induced by A. actinomycetemcomitans in BMMs compared with controls. Additionally, FTY720 attenuated p-PI3K, p-Akt, and p-ERK expressions induced by A. actinomycetemcomitans. Furthermore, FTY720 suppressed osteoclastogenesis in bone marrow-derived pre-osteoclasts with or without bacterial stimulation and reduced the mRNA levels of Nfatc1, Ctsk, Acp5, and Oscar, but not RANKL in bone marrow-derived pre-osteoclasts.
Conclusion: FTY720 inhibited proinflammatory cytokine production and suppressed osteoclastogenesis, supporting FTY720 as a potential therapy for inflammatory bone loss diseases.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

