The Neurogenic Potential of Astrocytes Is Regulated by Inflammatory Signals
- PMID: 26138449
- PMCID: PMC4937102
- DOI: 10.1007/s12035-015-9296-x
The Neurogenic Potential of Astrocytes Is Regulated by Inflammatory Signals
Abstract
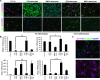
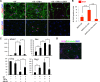
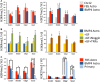
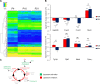
Although the adult brain contains neural stem cells (NSCs) that generate new neurons throughout life, these astrocyte-like populations are restricted to two discrete niches. Despite their terminally differentiated phenotype, adult parenchymal astrocytes can re-acquire NSC-like characteristics following injury, and as such, these 'reactive' astrocytes offer an alternative source of cells for central nervous system (CNS) repair following injury or disease. At present, the mechanisms that regulate the potential of different types of astrocytes are poorly understood. We used in vitro and ex vivo astrocytes to identify candidate pathways important for regulation of astrocyte potential. Using in vitro neural progenitor cell (NPC)-derived astrocytes, we found that exposure of more lineage-restricted astrocytes to either tumor necrosis factor alpha (TNF-α) (via nuclear factor-κB (NFκB)) or the bone morphogenetic protein (BMP) inhibitor, noggin, led to re-acquisition of NPC properties accompanied by transcriptomic and epigenetic changes consistent with a more neurogenic, NPC-like state. Comparative analyses of microarray data from in vitro-derived and ex vivo postnatal parenchymal astrocytes identified several common pathways and upstream regulators associated with inflammation (including transforming growth factor (TGF)-β1 and peroxisome proliferator-activated receptor gamma (PPARγ)) and cell cycle control (including TP53) as candidate regulators of astrocyte phenotype and potential. We propose that inflammatory signalling may control the normal, progressive restriction in potential of differentiating astrocytes as well as under reactive conditions and represent future targets for therapies to harness the latent neurogenic capacity of parenchymal astrocytes.
Keywords: Astrocytes; Epigenetic; Inflammation; NFκB; Neural stem cells; Noggin.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

