Transportability of confined field trial data from cultivation to import countries for environmental risk assessment of genetically modified crops
- PMID: 26138875
- PMCID: PMC4639567
- DOI: 10.1007/s11248-015-9892-6
Transportability of confined field trial data from cultivation to import countries for environmental risk assessment of genetically modified crops
Abstract
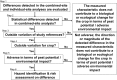
Requirement of in-country confined field trials for genetically modified (GM) crops prior to unrestricted release is well-established among countries with domestic regulations for the cultivation approval of GM crops. However, the requirement of in-country confined field trials is not common in countries where the scope of the application does not include cultivation. Nonetheless, Japan and China request in-country confined field trials for GM crops which are intended only for use as food, feed and processing. This paper considers the transportability of confined field trial data from cultivation countries (e.g. United States, Canada, and South American countries) to import countries like Japan for the environmental risk assessment of GM crops by reviewing: (1) the purpose of confined field trial assessment, (2) weediness potential, defined as "an ability to establish and persist in an unmanaged area that is frequently disturbed by human activity", of host crops, and (3) reliability of the confined field trial data obtained from cultivation countries. To review the reliability of the confined field data obtained in the US, this paper describes actual examples of three confined field trials of approved GM corn events conducted both in the US and Japan. Based on the above considerations, this paper concludes that confined field data of GM corn and cotton is transportable from cultivation countries to importing countries (e.g. from the US to Japan), regardless of the characteristics of the inserted gene(s). In addition, this paper advocates harmonization of protocols for confined field trials to facilitate more efficient data transportability across different geographies.
Keywords: Confined field trial; Data transportability; Environmental risk assessment; Genetically modified crops; Weediness potential.
Figures
Similar articles
-
Consideration of familiarity accumulated in the confined field trials for environmental risk assessment of genetically modified soybean (Glycine max) in Japan.Transgenic Res. 2020 Apr;29(2):229-242. doi: 10.1007/s11248-020-00193-z. Epub 2020 Jan 29. Transgenic Res. 2020. PMID: 31997144 Free PMC article.
-
Trends in global approvals of biotech crops (1992-2014).GM Crops Food. 2015;6(3):150-66. doi: 10.1080/21645698.2015.1056972. Epub 2015 Jun 3. GM Crops Food. 2015. PMID: 26039675 Free PMC article.
-
Do confined field trials add value for the environment risk assessment of genetically modified Brassica napus L. in Japan?Transgenic Res. 2025 Jan 7;34(1):6. doi: 10.1007/s11248-024-00425-6. Transgenic Res. 2025. PMID: 39777564 Free PMC article.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Innovative farmers and regulatory gatekeepers: Genetically modified crops regulation and adoption in developing countries.GM Crops Food. 2016 Jan 2;7(1):1-11. doi: 10.1080/21645698.2016.1151989. GM Crops Food. 2016. PMID: 26954893 Free PMC article. Review.
Cited by
-
Paraguay's Path Toward the Simplification of Procedures in the Approval of GE Crops.Front Bioeng Biotechnol. 2020 Aug 18;8:1023. doi: 10.3389/fbioe.2020.01023. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974329 Free PMC article.
-
Consideration of familiarity accumulated in the confined field trials for environmental risk assessment of genetically modified soybean (Glycine max) in Japan.Transgenic Res. 2020 Apr;29(2):229-242. doi: 10.1007/s11248-020-00193-z. Epub 2020 Jan 29. Transgenic Res. 2020. PMID: 31997144 Free PMC article.
-
A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment.Biology (Basel). 2021 Dec 3;10(12):1264. doi: 10.3390/biology10121264. Biology (Basel). 2021. PMID: 34943179 Free PMC article. Review.
-
Assessment of potential impacts associated with gene flow from transgenic hybrids to Mexican maize landraces.Transgenic Res. 2019 Dec;28(5-6):509-523. doi: 10.1007/s11248-019-00160-3. Epub 2019 Jun 27. Transgenic Res. 2019. PMID: 31250247 Free PMC article.
-
Environmental risk assessment of transgenic miraculin-accumulating tomato in a confined field trial in Japan.Plant Biotechnol (Tokyo). 2021 Dec 25;38(4):421-431. doi: 10.5511/plantbiotechnology.21.1021a. Plant Biotechnol (Tokyo). 2021. PMID: 35087307 Free PMC article.
References
-
- Anderson WP. Weed science: principles and applications. 3. St. Paul: West Publishing Company; 1996. Weed ecology; pp. 27–38.
-
- APHIS (2015) Petitions for determination of nonregulated status. http://www.aphis.usda.gov/biotechnology/petitions_table_pending.shtml. Accessed 15 Apr 2015
-
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York: Academic Press; 1965. pp. 147–168.
-
- Beard BH, Knowles PF. Frequency of cross-pollination of soybeans after seed irradiation. Crop Sci. 1971;11:489–492. doi: 10.2135/cropsci1971.0011183X001100040008x. - DOI
-
- Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M, Abad M, Kumar G, Salvador S, D’Ordine R, Navarro S, Back S, Fernandes M, Targolli J, Dasgupta S, Bonin C, Luethy MH, Heard JE. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol. 2008;147(2):446–455. doi: 10.1104/pp.108.118828. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical