DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers
- PMID: 26138979
- PMCID: PMC4672004
- DOI: 10.1126/science.aaa8651
DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers
Abstract
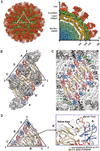
There are four closely-related dengue virus (DENV) serotypes. Infection with one serotype generates antibodies that may cross-react and enhance infection with other serotypes in a secondary infection. We demonstrated that DENV serotype 2 (DENV2)-specific human monoclonal antibody (HMAb) 2D22 is therapeutic in a mouse model of antibody-enhanced severe dengue disease. We determined the cryo-electron microscopy (cryo-EM) structures of HMAb 2D22 complexed with two different DENV2 strains. HMAb 2D22 binds across viral envelope (E) proteins in the dimeric structure, which probably blocks the E protein reorganization required for virus fusion. HMAb 2D22 "locks" two-thirds of or all dimers on the virus surface, depending on the strain, but neutralizes these DENV2 strains with equal potency. The epitope defined by HMAb 2D22 is a potential target for vaccines and therapeutics.
Copyright © 2015, American Association for the Advancement of Science.
Figures



Comment in
-
Dengue virus: Bumps in the road to therapeutic antibodies.Nature. 2015 Aug 20;524(7565):295-6. doi: 10.1038/524295a. Nature. 2015. PMID: 26289200 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

