Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep
- PMID: 26139099
- PMCID: PMC4566940
- DOI: 10.1096/fj.15-272013
Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep
Abstract
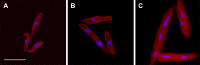
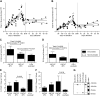
Studies in altricial rodents attribute dramatic changes in perinatal cardiomyocyte growth, maturation, and attrition to stimuli associated with birth. Our purpose was to determine whether birth is a critical trigger controlling perinatal cardiomyocyte growth, maturation and attrition in a precocial large mammal, sheep (Ovis aries). Hearts from 0-61 d postnatal lambs were dissected or enzymatically dissociated. Cardiomyocytes were measured by micromorphometry, cell cycle activity assessed by immunohistochemistry, and nuclear number counted after DNA staining. Integration of this new data with published fetal data from our laboratory demonstrate that a newly appreciated >30% decrease in myocyte number occurred in the last 10 d of gestation (P < 0.0005) concomitant with an increase in cleaved poly (ADP-ribose) polymerase 1 (P < 0.05), indicative of apoptosis. Bisegmental linear regressions show that most changes in myocyte growth kinetics occur before birth (median = 15.2 d; P < 0.05). Right ventricular but not left ventricular cell number increases in the neonate, by 68% between birth and 60 d postnatal (P = 0.028). We conclude that in sheep few developmental changes in cardiomyocytes result from birth, excepting the different postnatal degrees of free wall hypertrophy between the ventricles. Furthermore, myocyte number is reduced in both ventricles immediately before term, but proliferation increases myocyte number in the neonatal right ventricle.
Keywords: apoptosis; cell number; fetus; neonate; proliferation.
© FASEB.
Figures




References
-
- Soonpaa M. H., Kim K. K., Pajak L., Franklin M., Field L. J. (1996) Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271, H2183–H2189 - PubMed
-
- Walsh S., Pontén A., Fleischmann B. K., Jovinge S. (2010) Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovasc. Res. 86, 365–373 - PubMed
-
- Jonker S. S., Zhang L., Louey S., Giraud G. D., Thornburg K. L., Faber J. J. (2007) Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J. Appl. Physiol. (1985) 102, 1130–1142 - PubMed
-
- Burrell J. H., Boyn A. M., Kumarasamy V., Hsieh A., Head S. I., Lumbers E. R. (2003) Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 274A, 952–961 - PubMed
-
- Adler C. P., Friedburg H., Herget G. W., Neuburger M., Schwalb H. (1996) Variability of cardiomyocyte DNA content, ploidy level and nuclear number in mammalian hearts. Virchows Arch. 429, 159–164 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

