Eicosanoid storm in infection and inflammation
- PMID: 26139350
- PMCID: PMC4606863
- DOI: 10.1038/nri3859
Eicosanoid storm in infection and inflammation
Erratum in
- Nat Rev Immunol. 2015 Nov;15(11):724
Abstract
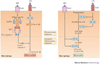
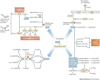
Controlled immune responses to infection and injury involve complex molecular signalling networks with coordinated and often opposing actions. Eicosanoids and related bioactive lipid mediators derived from polyunsaturated fatty acids constitute a major bioactive lipid network that is among the most complex and challenging pathways to map in a physiological context. Eicosanoid signalling, similar to cytokine signalling and inflammasome formation, has primarily been viewed as a pro-inflammatory component of the innate immune response; however, recent advances in lipidomics have helped to elucidate unique eicosanoids and related docosanoids with anti-inflammatory and pro-resolution functions. This has advanced our overall understanding of the inflammatory response and its therapeutic implications. The induction of a pro-inflammatory and anti-inflammatory eicosanoid storm through the activation of inflammatory receptors by infectious agents is reviewed here.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

