Biochemical Computation for Spine Structural Plasticity
- PMID: 26139370
- PMCID: PMC4722820
- DOI: 10.1016/j.neuron.2015.05.043
Biochemical Computation for Spine Structural Plasticity
Abstract
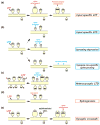
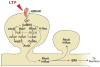
The structural plasticity of dendritic spines is considered to be essential for various forms of synaptic plasticity, learning, and memory. The process is mediated by a complex signaling network consisting of numerous species of molecules. Furthermore, the spatiotemporal dynamics of the biochemical signaling are regulated in a complicated manner because of geometrical restrictions from the unique morphology of the dendritic branches and spines. Recent advances in optical techniques have enabled the exploration of the spatiotemporal aspects of the signal regulations in spines and dendrites and have provided many insights into the principle of the biochemical computation that underlies spine structural plasticity.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. - PubMed
-
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature reviews Neuroscience. 2008;9:387. - PubMed
-
- Adams JP, Dudek SM. Late-phase long-term potentiation: getting to the nucleus. Nature reviews Neuroscience. 2005;6:737–743. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

