Structural Basis for the Interconversion of Maltodextrins by MalQ, the Amylomaltase of Escherichia coli
- PMID: 26139606
- PMCID: PMC4571864
- DOI: 10.1074/jbc.M115.667337
Structural Basis for the Interconversion of Maltodextrins by MalQ, the Amylomaltase of Escherichia coli
Abstract
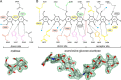
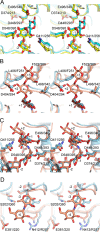
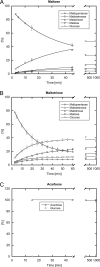
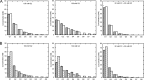
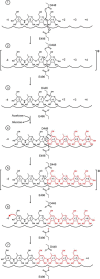
Amylomaltase MalQ is essential for the metabolism of maltose and maltodextrins in Escherichia coli. It catalyzes transglycosylation/disproportionation reactions in which glycosyl or dextrinyl units are transferred among linear maltodextrins of various lengths. To elucidate the molecular basis of transglycosylation by MalQ, we have determined three crystal structures of this enzyme, i.e. the apo-form, its complex with maltose, and an inhibitor complex with the transition state analog acarviosine-glucose-acarbose, at resolutions down to 2.1 Å. MalQ represents the first example of a mesophilic bacterial amylomaltase with known structure and exhibits an N-terminal extension of about 140 residues, in contrast with previously described thermophilic enzymes. This moiety seems unique to amylomaltases from Enterobacteriaceae and folds into two distinct subdomains that associate with different parts of the catalytic core. Intriguingly, the three MalQ crystal structures appear to correspond to distinct states of this enzyme, revealing considerable conformational changes during the catalytic cycle. In particular, the inhibitor complex highlights the requirement of both a 3-OH group and a 4-OH group (or α1-4-glycosidic bond) at the acceptor subsite +1 for the catalytically competent orientation of the acid/base catalyst Glu-496. Using an HPLC-based MalQ enzyme assay, we could demonstrate that the equilibrium concentration of maltodextrin products depends on the length of the initial substrate; with increasing numbers of glycosidic bonds, less glucose is formed. Thus, both structural and enzymatic data are consistent with the extremely low hydrolysis rates observed for amylomaltases and underline the importance of MalQ for the metabolism of maltodextrins in E. coli.
Keywords: Escherichia coli (E. coli); acarbose; carbohydrate metabolism; enzymatically derived inhibitor; enzyme mechanism; maltose/maltodextrin metabolism; protein crystallization; x-ray crystallography.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
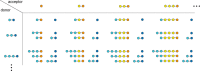

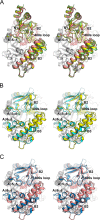
References
-
- Freundlieb S., Ehmann U., Boos W. (1988) Facilitated diffusion of p-nitrophenyl-α-d-maltohexaoside through the outer membrane of Escherichia coli. Characterization of LamB as a specific and saturable channel for maltooligosaccharides. J. Biol. Chem. 263, 314–320 - PubMed
-
- Becker S., Palm D., Schinzel R. (1994) Dissecting differential binding in the forward and reverse reaction of Escherichia coli maltodextrin phosphorylase using 2-deoxyglucosyl substrates. J. Biol. Chem. 269, 2485–2490 - PubMed
-
- O'Reilly M., Watson K. A., Schinzel R., Palm D., Johnson L. N. (1997) Oligosaccharide substrate binding in Escherichia coli maltodextrin phosphorylase. Nat. Struct. Biol. 4, 405–412 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

