Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer
- PMID: 26140237
- PMCID: PMC4485803
- DOI: 10.1080/2162402X.2015.1014242
Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer
Abstract
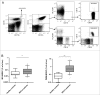
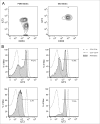
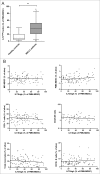
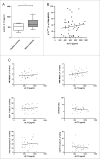
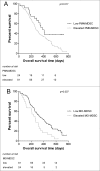
Myeloid-derived suppressor cells (MDSCs) play an important role in immune suppression and accumulate under pathologic conditions such as cancer and chronic inflammation. They comprise a heterogeneous population of immature myeloid cells that exert their immunosuppressive function via a variety of mechanisms. Immunoglobulin-like transcript 3 (ILT3) is a receptor containing immunoreceptor tyrosine-based inhibition motifs (ITIMs) that can be expressed on antigen-presenting cells and is an important regulator of dendritic cell tolerance. ILT3 exists in a membrane-bound and a soluble form and can interact with a yet unidentified ligand on T cells and thereby induce T-cell anergy, regulatory T cells, or T suppressor cells. In this study, we analyzed freshly isolated peripheral blood mononuclear cells (PBMCs) of 105 patients with non-small cell lung cancer and 20 healthy controls and demonstrated for the first time that ILT3 is expressed on MDSCs. We show that increased levels of circulating MDSCs correlate with reduced survival. On the basis of ILT3 cell surface expression, an ILT3low and ILT3high population of polymorphonuclear (PMN)-MDSCs could be distinguished. Interestingly, in line with the immunosuppressive function of ILT3 on dendritic cells, patients with an increased proportion of PMN-MDSCs and an increased fraction of the ILT3high subset had a shorter median survival than patients with elevated PMN-MDSC and a smaller ILT3high fraction. No correlation between the ILT3high subset and other immune variables was found. ILT3 expressed on MDSCs might reflect a previously unknown mechanism by which this cell population induces immune suppression and could therefore be an attractive target for immune intervention.
Keywords: APC, antigen-presenting cell; CD85k; DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; HC, healthy control; ILT3, immunoglobulin-like transcript 3; LILRB4; LIR-5; MDSC, myeloid-derived suppressor cell; MFI, mean fluorescence intensity; MO-MDSC, monocytic MDSC; NFκB, nuclear factor κB; NSCLC, non-small cell lung carcinoma; PBMC, peripheral blood mononuclear cell; PMN-MDSC, polymorphonuclear MDSC; Treg, regulatory T cell; Ts, T suppressor cell; immune suppression; immunoglobulin-like transcript 3; myeloid-derived suppressor cells; non-small cell lung cancer; overall survival; sILT3, soluble ILT3.
Figures
Similar articles
-
ILT3 (LILRB4) Promotes the Immunosuppressive Function of Tumor-Educated Human Monocytic Myeloid-Derived Suppressor Cells.Mol Cancer Res. 2021 Apr;19(4):702-716. doi: 10.1158/1541-7786.MCR-20-0622. Epub 2020 Dec 28. Mol Cancer Res. 2021. PMID: 33372059
-
Decidua-derived granulocyte macrophage colony-stimulating factor induces polymorphonuclear myeloid-derived suppressor cells from circulating CD15+ neutrophils.Hum Reprod. 2020 Dec 1;35(12):2677-2691. doi: 10.1093/humrep/deaa217. Hum Reprod. 2020. PMID: 33067638
-
LILRB4 promotes tumor metastasis by regulating MDSCs and inhibiting miR-1 family miRNAs.Oncoimmunology. 2022 Apr 5;11(1):2060907. doi: 10.1080/2162402X.2022.2060907. eCollection 2022. Oncoimmunology. 2022. PMID: 35402083 Free PMC article.
-
Immunomodulatory effects of myeloid-derived suppressor cells in diseases: Role in cancer and infections.Immunobiology. 2018 Apr-May;223(4-5):432-442. doi: 10.1016/j.imbio.2017.07.001. Epub 2017 Jul 4. Immunobiology. 2018. PMID: 29246400 Review.
-
Roles of Myeloid-Derived Suppressor Cell Subpopulations in Autoimmune Arthritis.Front Immunol. 2018 Dec 4;9:2849. doi: 10.3389/fimmu.2018.02849. eCollection 2018. Front Immunol. 2018. PMID: 30564242 Free PMC article. Review.
Cited by
-
Immunotherapy for Metastatic Non-Small Cell Lung Cancer: Therapeutic Advances and Biomarkers.Curr Oncol. 2023 Feb 16;30(2):2366-2387. doi: 10.3390/curroncol30020181. Curr Oncol. 2023. PMID: 36826142 Free PMC article. Review.
-
Host-Related Factors as Targetable Drivers of Immunotherapy Response in Non-Small Cell Lung Cancer Patients.Front Immunol. 2022 Jul 6;13:914890. doi: 10.3389/fimmu.2022.914890. eCollection 2022. Front Immunol. 2022. PMID: 35874749 Free PMC article. Review.
-
LncRNAs has been identified as regulators of Myeloid-derived suppressor cells in lung cancer.Front Immunol. 2023 Feb 2;14:1067520. doi: 10.3389/fimmu.2023.1067520. eCollection 2023. Front Immunol. 2023. PMID: 36817434 Free PMC article. Review.
-
Prostaglanin-E2 Potentiates the Suppressive Functions of Human Mononuclear Myeloid-Derived Suppressor Cells and Increases Their Capacity to Expand IL-10-Producing Regulatory T Cell Subsets.Front Immunol. 2019 Mar 18;10:475. doi: 10.3389/fimmu.2019.00475. eCollection 2019. Front Immunol. 2019. PMID: 30936876 Free PMC article.
-
Myeloid-derived suppressor cells-new and exciting players in lung cancer.J Hematol Oncol. 2020 Jan 31;13(1):10. doi: 10.1186/s13045-020-0843-1. J Hematol Oncol. 2020. PMID: 32005273 Free PMC article. Review.
References
-
- Bremnes RM, Al-Shibli K, Donnem T, Sirera R, Al-Saad S, Andersen S, Stenvold H, Camps C, Busund LT. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011; 6:824-33; PMID:; http://dx.doi.org/10.1097/JTO.0b013e3182037b76 - DOI - PubMed
-
- Heuvers ME, Aerts JG, Cornelissen R, Groen H, Hoogsteden HC, Hegmans JP. Patient-tailored modulation of the immune system may revolutionize future lung cancer treatment. BMC Cancer 2012; 12:580; PMID:; http://dx.doi.org/10.1186/1471-2407-12-580 - DOI - PMC - PubMed
-
- Jiang JW, Guo WJ, Liang XH. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Human Immunol 2014; 75:1128-37; PMID:; http://dx.doi.org/10.1016/j.humimm.2014.09.025 - DOI - PubMed
-
- Ortiz ML, Lu L, Ramachandran I, Gabrilovich DI. Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res 2014; 2:50-8; PMID:; http://dx.doi.org/10.1158/2326-6066.CIR-13-0129 - DOI - PMC - PubMed
-
- Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin Cytom 2014; PMID:; http://dx.doi.org/10.1002/cyto.b.21206 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
