Cross-talk between tumors can affect responses to therapy
- PMID: 26140251
- PMCID: PMC4485781
- DOI: 10.4161/2162402X.2014.975572
Cross-talk between tumors can affect responses to therapy
Abstract
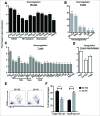
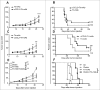
Advanced stages of cancer often involve multiple tumors in different locations in the body. These tumors are associated with a microenvironment that can influence tumor responses to immunotherapy. Whether tumors and their disparate microenvironment can interact together at distance in a multiple tumor setting, through a form of cross-talk, and affect their responses to immunotherapy has never been described. Our study investigated the cross-talk between two tumors with disparate microenvironments in a mouse model. We demonstrated that immunosuppressive visceral tumors could influence distant subcutaneous (SC) tumors to render them resistant to immunotherapy. We observed distinct modifications in the SC tumor microenvironment following cross-talk with kidney tumors that exhibit a type-2 macrophage-related immunosuppressive microenvironment. Indeed, when a concomitant kidney tumor was present in the mouse, the SC tumors were highly infiltrated with M2 macrophages and had a reduced T cell and NK cell effector immune profile. Finally, blocking the M2-associated chemokine CCL2 or depleting macrophages, significantly improved the effect of immunotherapy on growth of SC tumors in the presence of concomitant kidney tumors. This work emphasizes the potential negative influence that a tumor, with a strong immunosuppressive microenvironment, can exert on distant tumors that would normally be treatment-responsive. This report may lead to a new vision of the prioritization in the treatment of advanced metastatic cancer.
Keywords: C-C motif ligand-2; CD; CCL2; CD137; CD40; DR5; T cell receptor.; clodronate; cluster of differentiation; IFNγ; immunosuppression; immunotherapy; interferon gamma; IH; interleukin; M2; intrahepatic; IK; intrakidney; IL; natural killer; SC; subcutaneous; TCR; tumor immunology; tumor microenvironment; type 2 macrophages; NK; type-2 macrophages.
Figures




Similar articles
-
Differential potency of regulatory T cell-mediated immunosuppression in kidney tumors compared to subcutaneous tumors.Oncoimmunology. 2014 Dec 21;3(11):e963395. doi: 10.4161/21624011.2014.963395. eCollection 2014 Nov. Oncoimmunology. 2014. PMID: 25941590 Free PMC article.
-
Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy.Mol Ther. 2014 Jan;22(1):18-27. doi: 10.1038/mt.2013.219. Epub 2013 Sep 19. Mol Ther. 2014. PMID: 24048441 Free PMC article.
-
Therapeutic gene modified cell based cancer vaccines.Gene. 2013 Aug 10;525(2):200-7. doi: 10.1016/j.gene.2013.03.056. Epub 2013 Apr 6. Gene. 2013. PMID: 23566846 Review.
-
Local Blockade of Interleukin 10 and C-X-C Motif Chemokine Ligand 12 with Nano-Delivery Promotes Antitumor Response in Murine Cancers.ACS Nano. 2018 Oct 23;12(10):9830-9841. doi: 10.1021/acsnano.8b00967. Epub 2018 Sep 28. ACS Nano. 2018. PMID: 30253648
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
Cited by
-
The Liver-Immunity Nexus and Cancer Immunotherapy.Clin Cancer Res. 2022 Jan 1;28(1):5-12. doi: 10.1158/1078-0432.CCR-21-1193. Epub 2021 Jul 20. Clin Cancer Res. 2022. PMID: 34285059 Free PMC article. Review.
-
Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells.PLoS One. 2017 Apr 25;12(4):e0175558. doi: 10.1371/journal.pone.0175558. eCollection 2017. PLoS One. 2017. PMID: 28441391 Free PMC article.
-
A Syngeneic Mouse Model of Metastatic Renal Cell Carcinoma for Quantitative and Longitudinal Assessment of Preclinical Therapies.J Vis Exp. 2017 Apr 12;(122):55080. doi: 10.3791/55080. J Vis Exp. 2017. PMID: 28448047 Free PMC article.
-
Tissue-Dependent Tumor Microenvironments and Their Impact on Immunotherapy Responses.Front Immunol. 2018 Jan 31;9:70. doi: 10.3389/fimmu.2018.00070. eCollection 2018. Front Immunol. 2018. PMID: 29445373 Free PMC article. Review.
-
A New Systemic Disease Mouse Model for Glioblastoma Capable of Single-Tumour-Cell Detection.Cells. 2024 Jan 19;13(2):192. doi: 10.3390/cells13020192. Cells. 2024. PMID: 38275817 Free PMC article.
References
-
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9:274-84; PMID:; http://dx.doi.org/10.1038/nrc2622 - DOI - PubMed
-
- Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO, Dickman PW, Hall P. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol 2007; 25:4210-6; PMID:; http://dx.doi.org/10.1200/JCO.2006.10.5056 - DOI - PubMed
-
- Singer EA, Vourganti S, Lin KY, Gupta GN, Pinto PA, Rastinehad AR, Linehan WM, Bratslavsky G. Outcomes of patients with surgically treated bilateral renal masses and a minimum of 10 years of followup. J Urol 2012; 188:2084-8; PMID:; http://dx.doi.org/10.1016/j.juro.2012.08.038 - DOI - PMC - PubMed
-
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27:5904-12; PMID:; http://dx.doi.org/10.1038/onc.2008.271 - DOI - PMC - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57-70; PMID:; http://dx.doi.org/10.1016/S0092-8674(00)81683-9 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
