Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses
- PMID: 26140594
- PMCID: PMC4526462
- DOI: 10.1016/j.cell.2015.06.027
Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses
Abstract
Stimuli that possess inherently rewarding or aversive qualities elicit emotional responses and also induce learning by imparting valence upon neutral sensory cues. Evidence has accumulated implicating the amygdala as a critical structure in mediating these processes. We have developed a genetic strategy to identify the representations of rewarding and aversive unconditioned stimuli (USs) in the basolateral amygdala (BLA) and have examined their role in innate and learned responses. Activation of an ensemble of US-responsive cells in the BLA elicits innate physiological and behavioral responses of different valence. Activation of this US ensemble can also reinforce appetitive and aversive learning when paired with differing neutral stimuli. Moreover, we establish that the activation of US-responsive cells in the BLA is necessary for the expression of a conditioned response. Neural representations of conditioned and unconditioned stimuli therefore ultimately connect to US-responsive cells in the BLA to elicit both innate and learned responses.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

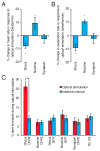
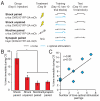
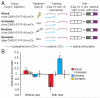
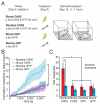


Comment in
-
Neuronal circuits: Connecting to innate knowledge.Nat Rev Neurosci. 2015 Aug;16(8):441. doi: 10.1038/nrn3999. Nat Rev Neurosci. 2015. PMID: 26189691 No abstract available.
References
-
- Amaral D, Price J, Pitkanen A, Carmichael S. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J, editor. Wiley-Liss; New York: 1992. pp. 1–66.
-
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

