Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2
- PMID: 26140667
- PMCID: PMC4742294
- DOI: 10.1111/bph.13237
Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2
Abstract
Background and purpose: Neutrophil elastase plays a crucial role in arthritis. Here, its potential in triggering joint inflammation and pain was assessed, and whether these effects were mediated by proteinase-activated receptor-2 (PAR2).
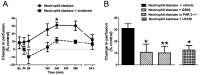
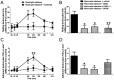
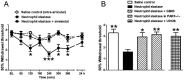
Experimental approach: Neutrophil elastase (5 μg) was injected into the knee joints of mice and changes in blood perfusion, leukocyte kinetics and paw withdrawal threshold were assessed. Similar experiments were performed in animals pretreated with the neutrophil elastase inhibitor sivelestat, the PAR2 antagonist GB83, the p44/42 MAPK inhibitor U0126 and in PAR2 receptor knockout (KO) mice. Neutrophil elastase activity was also evaluated in arthritic joints by fluorescent imaging and sivelestat was assessed for anti-inflammatory and analgesic properties.
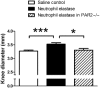
Key results: Intra-articular injection of neutrophil elastase caused an increase in blood perfusion, leukocyte kinetics and a decrease in paw withdrawal threshold. Sivelestat treatment suppressed this effect. The PAR2 antagonist GB83 reversed neutrophil elastase-induced synovitis and pain and these responses were also attenuated in PAR2 KO mice. The MAPK inhibitor U0126 also blocked neutrophil elastase-induced inflammation and pain. Active neutrophil elastase was increased in acutely inflamed knees as shown by an activatable fluorescent probe. Sivelestat appeared to reduce neutrophil elastase activity, but had only a moderate anti-inflammatory effect in this model.
Conclusions and implications: Neutrophil elastase induced acute inflammation and pain in knee joints of mice. These changes are PAR2-dependent and appear to involve activation of a p44/42 MAPK pathway. Blocking neutrophil elastase, PAR2 and p44/42 MAPK activity can reduce inflammation and pain, suggesting their utility as therapeutic targets.
© 2015 The British Pharmacological Society.
Figures

References
-
- Adeyemi EO, Campos LB, Loizou S, Walport MJ, Hodgson HJF (1990). Plasma lactoferrin and neutrophil elastase in rheumatoid arthritis and systemic lupus erythematosus. Br J Rheumatol 29: 15–20. - PubMed
-
- Andruski B, McCafferty DM, Ignacy T, Millen B, McDougall JJ (2008). Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am J Physiol Regul Integr Comp Physiol 295: R814–R820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

