Continuous distending pressure for respiratory distress in preterm infants
- PMID: 26141572
- PMCID: PMC7133489
- DOI: 10.1002/14651858.CD002271.pub2
Continuous distending pressure for respiratory distress in preterm infants
Update in
-
Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants.Cochrane Database Syst Rev. 2020 Oct 15;10(10):CD002271. doi: 10.1002/14651858.CD002271.pub3. Cochrane Database Syst Rev. 2020. PMID: 33058208 Free PMC article.
Abstract
Background: Respiratory distress syndrome (RDS) is the single most important cause of morbidity and mortality in preterm infants. In infants with progressive respiratory insufficiency, intermittent positive pressure ventilation (IPPV) with surfactant is the standard treatment for the condition, but it is invasive, potentially resulting in airway and lung injury. Continuous distending pressure (CDP) has been used for the prevention and treatment of RDS, as well as for the prevention of apnoea, and in weaning from IPPV. Its use in the treatment of RDS might reduce the need for IPPV and its sequelae.
Objectives: To determine the effect of continuous distending pressure (CDP) on the need for IPPV and associated morbidity in spontaneously breathing preterm infants with respiratory distress.Subgroup analyses were planned on the basis of birth weight (> or < 1000 or 1500 g), gestational age (groups divided at about 28 weeks and 32 weeks), methods of application of CDP (i.e. CPAP and CNP), application early versus late in the course of respiratory distress and high versus low pressure CDP and application of CDP in tertiary compared with non-tertiary hospitals, with the need for sensitivity analysis determined by trial quality.At the 2008 update, the objectives were modified to include preterm infants with respiratory failure.
Search methods: We used the standard search strategy of the Neonatal Review Group. This included searches of the Oxford Database of Perinatal Trials, the Cochrane Central Register of Controlled Trials (CENTRAL, 2015 Issue 4), MEDLINE (1966 to 30 April 2015) and EMBASE (1980 to 30 April 2015) with no language restriction, as well as controlled-trials.com, clinicaltrials.gov and the International Clinical Trials Registry Platform of the World Health Organization (WHO).
Selection criteria: All random or quasi-random trials of preterm infants with respiratory distress were eligible. Interventions were continuous distending pressure including continuous positive airway pressure (CPAP) by mask, nasal prong, nasopharyngeal tube or endotracheal tube, or continuous negative pressure (CNP) via a chamber enclosing the thorax and the lower body, compared with spontaneous breathing with oxygen added as necessary.
Data collection and analysis: We used standard methods of The Cochrane Collaboration and its Neonatal Review Group, including independent assessment of trial quality and extraction of data by each review author.
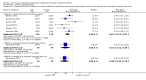
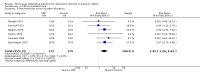
Main results: We included six studies involving 355 infants - two using face mask CPAP, two CNP, one nasal CPAP and one both CNP (for less ill babies) and endotracheal CPAP (for sicker babies). For this update, we included no new trials.Continuous distending pressure (CDP) is associated with lower risk of treatment failure (death or use of assisted ventilation) (typical risk ratio (RR) 0.65, 95% confidence interval (CI) 0.52 to 0.81; typical risk difference (RD) -0.20, 95% CI -0.29 to -0.10; number needed to treat for an additional beneficial outcome (NNTB) 5, 95% CI 4 to 10; six studies; 355 infants), lower overall mortality (typical RR 0.52, 95% CI 0.32 to 0.87; typical RD -0.15, 95% CI -0.26 to -0.04; NNTB 7, 95% CI 4 to 25; six studies; 355 infants) and lower mortality in infants with birth weight above 1500 g (typical RR 0.24, 95% CI 0.07 to 0.84; typical RD -0.28, 95% CI -0.48 to -0.08; NNTB 4, 95% CI 2.00 to 13.00; two studies; 60 infants). Use of CDP is associated with increased risk of pneumothorax (typical RR 2.64, 95% CI 1.39 to 5.04; typical RD 0.10, 95% CI 0.04 to 0.17; number needed to treat for an additional harmful outcome (NNTH) 17, 95% CI 17.00 to 25.00; six studies; 355 infants). We found no difference in bronchopulmonary dysplasia (BPD), defined as oxygen dependency at 28 days (three studies, 260 infants), as well as no difference in outcome at nine to 14 years (one study, 37 infants).
Authors' conclusions: In preterm infants with respiratory distress, the application of CDP as CPAP or CNP is associated with reduced respiratory failure and mortality and an increased rate of pneumothorax. Four out of six of these trials were done in the 1970s. Therefore, the applicability of these results to current practice is difficult to assess. Further research is required to determine the best mode of administration.
Conflict of interest statement
None.
Figures





































Update of
-
Continuous distending pressure for respiratory distress syndrome in preterm infants.Cochrane Database Syst Rev. 2002;(2):CD002271. doi: 10.1002/14651858.CD002271. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2015 Jul 04;(7):CD002271. doi: 10.1002/14651858.CD002271.pub2. PMID: 12076445 Updated.
References
References to studies included in this review
Belenky 1976 {published and unpublished data}
-
- Belenky DA, Orr RJ, Woodrum DE, Hodson WA. Is continuous transpulmonary pressure better than conventional respiratory management of hyaline membrane disease? A controlled study. Pediatrics 1976;58(6):800‐8. - PubMed
Buckmaster 2007 {published and unpublished data}
-
- Buckmaster AG, Gaston A, Wright IMR, Foster JP, Henderson‐Smart DJ. Continuous positive airway pressure therapy for infants with respiratory distress in non tertiary care centers: a randomized controlled trial. Pediatrics 2007;120(3):509‐18. - PubMed
Durbin 1976 {published data only}
Fanaroff 1973 {published data only}
-
- Fanaroff AA, Cha CC, Sosa R, Crumrine RS, Klaus MH. Controlled trial of continuous negative external pressure in the treatment of severe respiratory distress syndrome. Journal of Pediatrics 1973;82(6):921‐8. - PubMed
Rhodes 1973 {published data only}
-
- Rhodes PG, Hall RT. Continuous positive airway pressure delivered by face mask in infants with the idiopathic respiratory distress syndrome: a controlled study. Pediatrics 1973;52(1):1‐5. - PubMed
Samuels 1996 {published data only}
-
- Samuels MP, Raine J, Wright T, Alexander JA, Lockyer K, Spencer A, et al. Continuous negative extrathoracic pressure in neonatal respiratory failure. Pediatrics 1996;98(6 Pt 1):1154‐60. - PubMed
-
- Telford K, Waters L, Vyas H, Manktelow BN, Draper ES, Marlow N. Outcome after neonatal continuous negative‐pressure ventilation: follow‐up assessment. Lancet 2006;367(9516):1080‐85. - PubMed
References to studies excluded from this review
Colnaghi 2008 {published data only}
-
- Colnaghi N, Matassa PG, Fumagalli M, Messina D, Mosca F. Pharyngeal pressure value using two continuous positive airway pressure devices. Archives of Disease in Childhood ‐ Fetal and Neonatal Edition 2008;93(4):F302‐4. - PubMed
Dunn 2011 {published data only}
-
- Dunn MS, Kaempf J, Klerk A, Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128(5):e1069‐76. [PUBMED: 22025591] - PubMed
Finer 2010 {published data only}
Morley 2008 {published data only}
-
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, for the COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New England Journal of Medicine 2008;358(7):700‐8. - PubMed
Pieper 2003 {published data only}
-
- Pieper CH, Smith J, Maree D, Pohl FC. Is nCPAP of value in extreme preterms with no access to neonatal intensive care?. Journal of Tropical Pediatrics 2003;49(3):148‐52. - PubMed
Rojas 2009 {published data only}
-
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009;123(1):137‐42. - PubMed
Sandri 2010 {published data only}
-
- Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010;124(6):e1402‐9. - PubMed
Swyer 1973 {published data only}
-
- Swyer PR, Bryan MH, Chance GW, McMurray SB, Olinsky A, Reilly B. Continuous pressure breathing in RDS: comparative trial of 3 methods. INSERM Paris 1973.
Tapia 2012 {published data only}
-
- Tapia JL, Urzua S, Bancalari A, Meritano J, Torres G, Fabres J, et al. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. Journal of Pediatrics 2012;161(1):75‐80.e1. [PUBMED: 22402568] - PubMed
Tooley 2003 {published data only}
-
- Tooley J, Dyke M. Randomized study of nasal continuous positive airway pressure in the preterm infant with respiratory distress syndrome. Acta Paediatrica 2003;92(10):1170‐4. - PubMed
Additional references
Bancalari 1992
-
- Bancalari E, Sinclair JC. Mechanical ventilation. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:200‐220.
Davis 2004
De Paoli 2008
-
- Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2008, issue 1. [DOI: 10.1002/14651858.CD002977.pub2; CD002977] - DOI - PMC - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org, Version 5.1.0 [updated March 2011].
Greenough 2004
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions [updated March 2011]. Version 5.1.0. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ho 2004
Narendran 2003
-
- Narendran V, Donovan EF, Hoath SB, Akinbi HT, Steichen JJ, Jobe AH. Early bubble CPAP and outcomes in ELBW preterm infants. Journal of Perinatology 2003;23(3):195‐9. - PubMed
Pape 1976
-
- Pape KE, Armstrong DL, Fitzharding PM. Central nervous system pathology associated with mask ventilation in the very low birth weight infant: a new etiology for intracerebellar hemorrhages. Pediatrics 1976;58(4):473‐83. - PubMed
Soll 2004
Subramaniam 2005
-
- Subramaniam P, Henderson‐Smart DJ, Davis PG. Prophylactic nasal continuous positive airways pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2005, issue 3. [DOI: 10.1002/14651858.CD001243.pub2; CD001243] - DOI - PubMed
Verder 1999
-
- Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics 1999;103(2):e24. - PubMed
References to other published versions of this review
Ho 2000
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

