Impact of amyloid β aggregate maturation on antibody treatment in APP23 mice
- PMID: 26141728
- PMCID: PMC4491274
- DOI: 10.1186/s40478-015-0217-z
Impact of amyloid β aggregate maturation on antibody treatment in APP23 mice
Abstract
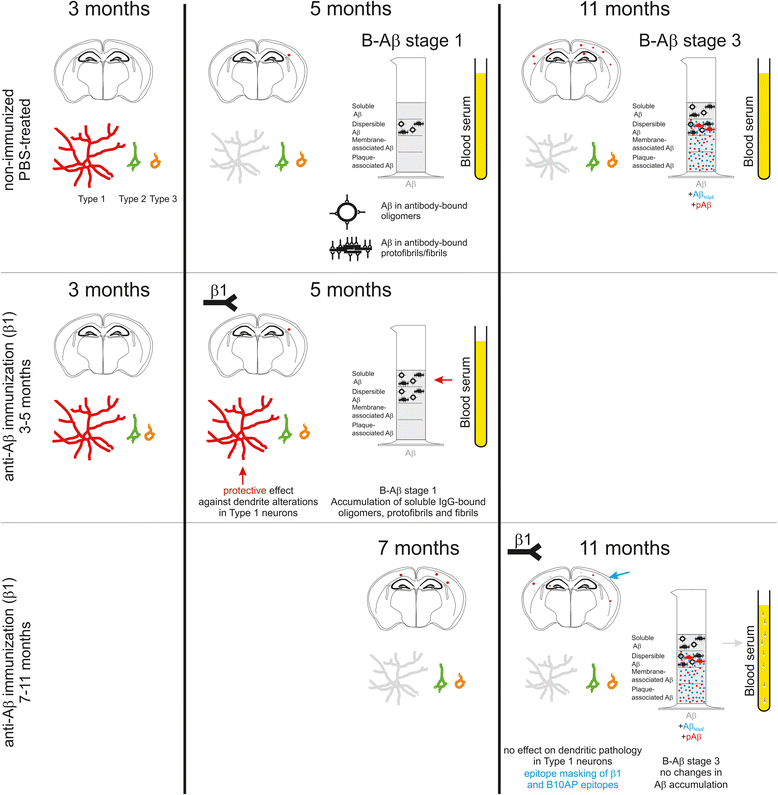
Introduction: The deposition of the amyloid β protein (Aβ) in the brain is a hallmark of Alzheimer's disease (AD). Removal of Aβ by Aβ-antibody treatment has been developed as a potential treatment strategy against AD. First clinical trials showed neither a stop nor a reduction of disease progression. Recently, we have shown that the formation of soluble and insoluble Aβ aggregates in the human brain follows a hierarchical sequence of three biochemical maturation stages (B-Aβ stages). To test the impact of the B-Aβ stage on Aβ immunotherapy, we treated transgenic mice expressing human amyloid precursor protein (APP) carrying the Swedish mutation (KM670/671NL; APP23) with the Aβ-antibody β1 or phosphate-buffered saline (PBS) beginning 1) at 3 months, before the onset of dendrite degeneration and plaque deposition, and 2) at 7 months, after the start of Aβ plaque deposition and dendrite degeneration.
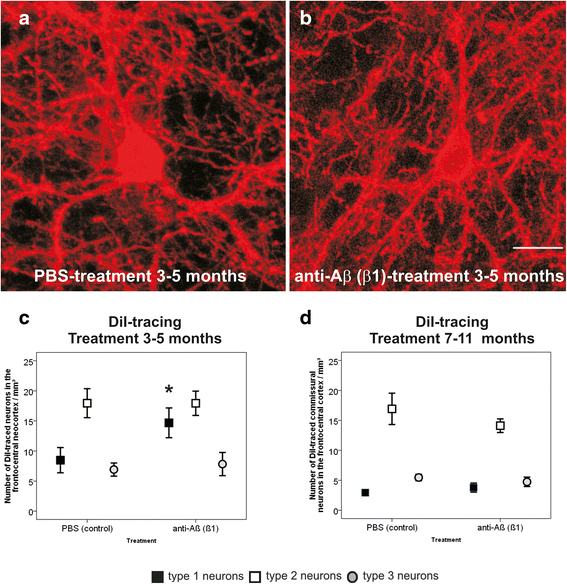
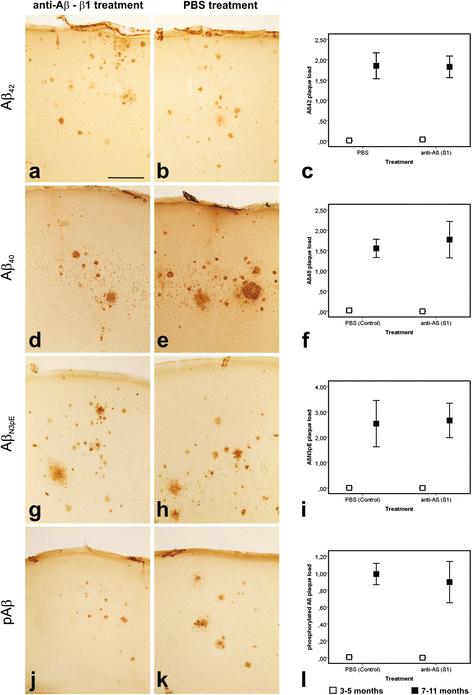
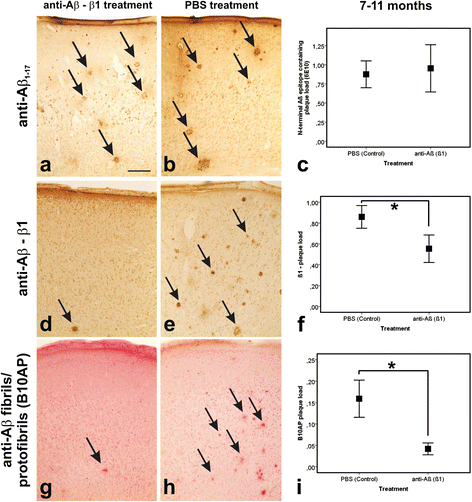
Results: At 5 months of age, first Aβ aggregates in APP23 brain consisted of non-modified Aβ (representing B-Aβ stage 1) whereas mature Aβ-aggregates containing N-terminal truncated, pyroglutamate-modified AβN3pE and phosphorylated Aβ (representing B-Aβ stage 3) were found at 11 months of age in both β1- and PBS-treated animals. Protective effects on commissural neurons with highly ramified dendritic trees were observed only in 3-month-old β1-treated animals sacrificed at 5 months. When treatment started at 7 months of age, no differences in the numbers of healthy commissural neurons were observed between β1- and PBS-treated APP23 mice sacrificed with 11 months.
Conclusions: Aβ antibody treatment was capable of protecting neurons from dendritic degeneration as long as Aβ aggregation was absent or represented B-Aβ stage 1 but had no protective or curative effect in later stages with mature Aβ aggregates (B-Aβ stage 3). These data indicate that the maturation stage of Aβ aggregates has impact on potential treatment effects in APP23 mice.
Figures




References
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9. doi: 10.1038/78682. - DOI - PubMed
-
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–7. doi: 10.1038/22124. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

