Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004-2012: a cohort study
- PMID: 26141729
- PMCID: PMC4491264
- DOI: 10.1186/s12913-015-0912-2
Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004-2012: a cohort study
Abstract
Background: The number of Human Immunodeficiency Virus (HIV) infected people eligible for initiation on antiretroviral Therapy (ART) is increasing. ART programmatic success requires that patients who are taking ART remain on treatment and are followed up regularly. This study investigated factors associated with being lost to follow-up, in a cohort of patients enrolled in a pharmacovigilance study in South Africa.
Methods: This was a retrospective observational cohort study performed at one of the Medunsa National Pharmacovigilance Centre's (MNPC) ART sentinel surveillance sites. Loss to Follow-up (LTFU) was defined as "a patient who had been followed up at the sentinel site, who had not had contact with the health facility for 180 days or more since their last recorded expected date of return or if there were 180 days or more between the expected date of return and the next clinic visit".
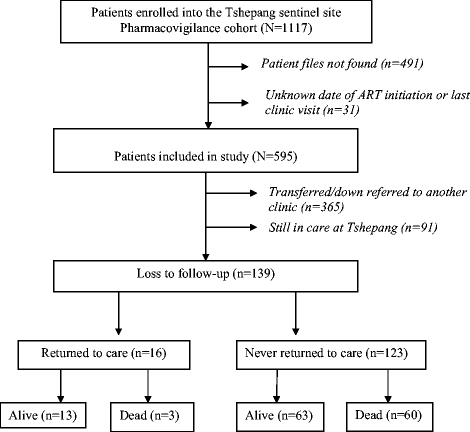
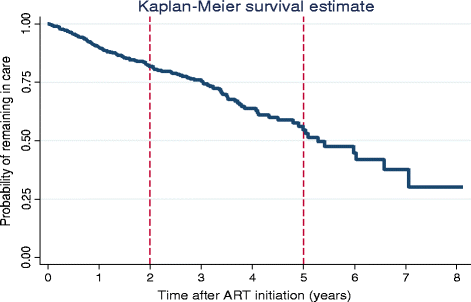
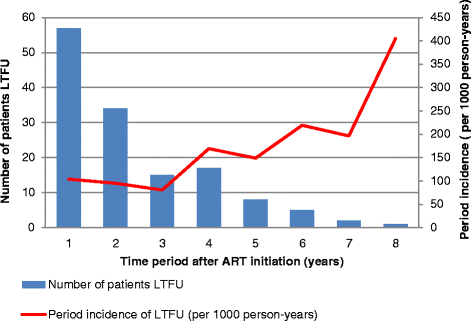
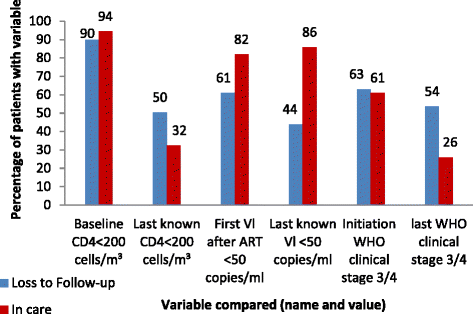
Results: Out of 595 patients, 65.5% (n = 390) were female and 23.4% (n = 139) were LTFU. The median time on ART before LTFU was 21.5 months (interquartile range: 12.9 - 34.7 months). The incidence rate of LTFU was 103 per 1000 person-years in the first year on ART and increased to 405 per 1000 person-years in the eighth year of taking ART. Factors associated with becoming LTFU included not having a committed partner (Adjusted Hazard Ratio (aHR): 2.9, 95% Confidence Interval (CI):1.19-6.97, p = 0.019), being self-employed (aHR: 13.9, 95% CI:2.81 - 69.06, p = 0.001), baseline CD4 count > 200 cells/ml (aHR: 3.8, 95% CI: 1.85-7.85, p < 0.001), detectable last known Viral Load (VL) (aHR: 3.6, 95% CI:1.98-6.52, p < 0.001) and a last known World Health Organisation clinical stage three or four (aHR: 2.0, 95% CI:1.22-3.27, p = 0.006). Patients that previously had an ART adverse event had a lower risk (aHR: 0.6, 95% CI: 0.38 - 0.99, p = 0.044) of becoming LTFU than those that had not.
Conclusion: The incidence rate of LTFU increases with additional years on ART. Intensified measures to improve patient retention on ART must be prioritised with increasing patient time on ART and in patients that are at increased risk of becoming lost to follow-up.
Figures
References
-
- UNAIDS. Global Health report: UNAIDS report on global AIDS epidemic. 2013. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Repo.... Accessed 13 Jun 2014.
-
- WHO. WHO fact sheet No 360. 2012. http://www.who.int/mediacentre/factsheets/fs360/en/index.html. Accessed 14 Oct 2013.
-
- UNAIDS: AIDS info Data. http://www.unaids.org/en/dataanalysis/datatools/aidsinfo/. Accessed 12 May 2015.
-
- Republic of South Africa. Global AIDS Response: Progress Report 2012. http://www.unaids.org/sites/default/files/country/documents//ce_ZA_Narra.... Accessed 5 Nov 2013.
-
- Wilson D, Cotton M, Bekker L, Meyers T, Venter F, Maartens G. Handbook of HIV Medicine. 2. Capetown: Oxford university Press Southern Africa (Pty) Ltd; 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

