Cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats
- PMID: 26141841
- PMCID: PMC4532610
- DOI: 10.1016/j.neuroscience.2015.06.050
Cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats
Abstract
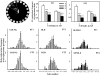
The hippocampus, a medial temporal lobe structure necessary for the formation of spatial memory, is particularly affected by both normal and pathologic aging. In previous studies, we observed a significant age-related increase in dopaminergic neuron loss in the hypothalamus and the substantia nigra of female rats, which becomes more conspicuous at extreme ages. Here, we extend our studies by assessing spatial memory in 4-6 month-old (young), 26-month-old (old) and 29-32-month-old (senile) Sprague-Dawley female rats as well as the age-related histopathological changes in their dorsal hippocampus. Age changes in spatial memory performance were assessed with a modified version of the Barnes maze test. We employed two probe trials (PTs), one and five days after training, respectively, in order to evaluate learning ability as well as short-term and longer-term spatial memory retention. A set of relevant hippocampal cell markers was also quantitated in the animals by means of an unbiased stereological approach. The results revealed that old rats perform better than senile rats in acquisition trials and young rats perform better than both aging groups. However, during short-term PT both aging groups showed a preserved spatial memory while in longer-term PT, spatial memory showed deterioration in both aged groups. Morphological analysis showed a marked decrease (94-97%) in doublecortin neuron number in the dentate gyrus in both aged groups and a reduction in glial fibrillary acidic protein-positive cell number in the stratum radiatum of aging rats. Astroglial process length and branching complexity decreased in aged rats. We conclude that while target-seeking activity and learning ability decrease in aged females, spatial memory only declines in the longer-term tests. The reduction in neuroblast number and astroglial arborescence complexity in the dorsal hippocampus are likely to play a role in the cognitive deficits of aging rats.
Keywords: Barnes maze; GFAP; aging; doublecortin; female-spatial memory; hippocampus.
Copyright © 2015 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



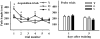
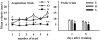


References
-
- Azcoitia I1, Perez-Martin M, Salazar V, Castillo C, Ariznavarreta C, Garcia-Segura LM, Tresguerres JA. Growth hormone prevents neuronal loss in the aged rat hippocampus. Neurobiol Aging. 2005;26:697–703. - PubMed
-
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. - PubMed
-
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34:29–39. - PubMed
-
- Barrett GL, Bennie A, Trieu J, Ping S, Tsafoulis C. The chronology of ag related spatial learning impairment in two rat strains, as tested by the Barnes maze. Behav Neurosci. 2009;123:533–538. - PubMed
-
- Begega A1, Cuesta M, Rubio S, Méndez M, Santín LJ, Arias JL. Functional Networks involved in spatial learning strategies in middle-aged rats. Neurobiol Learn Mem. 2012;97:346–353. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

