Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain
- PMID: 26142314
- PMCID: PMC4542288
- DOI: 10.1242/bio.012773
Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain
Erratum in
-
Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain.Biol Open. 2016 Feb 15;5(2):204. doi: 10.1242/bio.016980. Biol Open. 2016. PMID: 26879935 Free PMC article. No abstract available.
Abstract
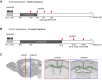
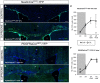
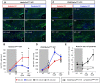
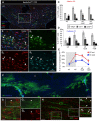
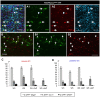
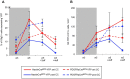
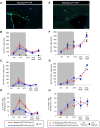
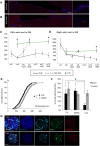
Myelin regeneration can occur in the brain following demyelination. Parenchymal oligodendrocyte progenitors (pOPC) are known to play a crucial role in this process. Neural stem cells (NSC) residing in the ventricular-subventricular zone (V-SVZ) also have the ability to generate oligodendrocytes but their contribution to endogenous myelin repair was so far considered to be negligible. Here, we addressed the relative contribution of pOPC and V-SVZ-derived neural progenitors (SVZdNP) to remyelination in cuprizone mouse models of acute or chronic corpus callosum (CC) demyelination. Using genetic tracing, we uncover an unexpected massive and precocious recruitment of SVZdNP in the anterior CC after acute demyelination. These cells very quickly adopt an oligodendrocytic fate and robustly generate myelinating cells as efficiently as pOPC do. In more posterior areas of the CC, SVZdNP recruitment is less important whereas pOPC contribute more, underlining a regionalization in the mobilization of these two cell populations. Strikingly, in a chronic model when demyelination insult is sustained in time, SVZdNP minimally contribute to myelin repair, a failure associated with a depletion of NSC and a drastic drop of progenitor cell proliferation in V-SVZ. In this context, pOPC remain reactive, and become the main contributors to myelin regeneration. Altogether our results highlight a region and context-dependent contribution of SVZdNP to myelin repair that can equal pOPC. They also raise the question of a possible exhaustion of V-SVZ proliferation potential in chronic pathologies.
Keywords: Adult neural stem cells; Myelin regeneration; Oligodendrocyte precursors; Subventricular zone.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
References
-
- Cusimano M., Biziato D., Brambilla E., Donega M., Alfaro-Cervello C., Snider S., Salani G., Pucci F., Comi G., Garcia-Verdugo J. M. et al. (2012). Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 135, 447-460. 10.1093/brain/awr339 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

