Recent progress in understanding coxsackievirus replication, dissemination, and pathogenesis
- PMID: 26142496
- PMCID: PMC4567421
- DOI: 10.1016/j.virol.2015.06.006
Recent progress in understanding coxsackievirus replication, dissemination, and pathogenesis
Abstract
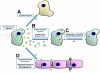
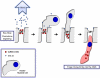
Coxsackieviruses (CVs) are relatively common viruses associated with a number of serious human diseases, including myocarditis and meningo-encephalitis. These viruses are considered cytolytic yet can persist for extended periods of time within certain host tissues requiring evasion from the host immune response and a greatly reduced rate of replication. A member of Picornaviridae family, CVs have been historically considered non-enveloped viruses - although recent evidence suggest that CV and other picornaviruses hijack host membranes and acquire an envelope. Acquisition of an envelope might provide distinct benefits to CV virions, such as resistance to neutralizing antibodies and efficient nonlytic viral spread. CV exhibits a unique tropism for progenitor cells in the host which may help to explain the susceptibility of the young host to infection and the establishment of chronic disease in adults. CVs have also been shown to exploit autophagy to maximize viral replication and assist in unconventional release from target cells. In this article, we review recent progress in clarifying virus replication and dissemination within the host cell, identifying determinants of tropism, and defining strategies utilized by the virus to evade the host immune response. Also, we will highlight unanswered questions and provide future perspectives regarding the potential mechanisms of CV pathogenesis.
Keywords: Autophagy; Cardiac progenitor cells; Coxsackievirus; Enterovirus; Meningoencephalitis; Microvesicles; Myocarditis; Neural progenitor cells; Picornavirus; Virus dissemination.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Abzug MJ. Presentation, diagnosis, and management of enterovirus infections in neonates. Paediatr.Drugs. 2004;6:1–10. - PubMed
-
- Ahn J, Jee Y, Seo I, Yoon SY, Kim D, Kim YK, Lee H. Primary neurons become less susceptible to coxsackievirus B5 following maturation: the correlation with the decreased level of CAR expression on cell surface. J.Med.Virol. 2008;80(3):434–440. - PubMed
-
- Alderman CP, Moritz CK, Ben-Tovim DI. Abnormal platelet aggregation associated with fluoxetine therapy. Ann.Pharmacother. 1992;26:1517–1519. - PubMed
-
- Alidjinou EK, Sane F, Bertin A, Caloone D, Hober D. Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine. Antiviral Res. 2015:10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

