Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes
- PMID: 26142898
- PMCID: PMC7104525
- DOI: 10.1002/14651858.CD003935.pub4
Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes
Update in
-
Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes.Cochrane Database Syst Rev. 2022 Apr 4;4(4):CD003935. doi: 10.1002/14651858.CD003935.pub5. Cochrane Database Syst Rev. 2022. PMID: 35377461 Free PMC article.
Abstract
Background: It has been unclear whether repeat dose(s) of prenatal corticosteroids are beneficial.
Objectives: To assess the effectiveness and safety of repeat dose(s) of prenatal corticosteroids.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (20 January 2015), searched reference lists of retrieved studies and contacted authors for further data.
Selection criteria: Randomised controlled trials of women who had already received a single course of corticosteroids seven or more days previously and considered still at risk of preterm birth.
Data collection and analysis: We assessed trial quality and extracted data independently.
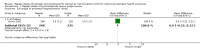
Main results: We included 10 trials (a total of 4733 women and 5700 babies) with low to moderate risk of bias. Treatment of women who remain at risk of preterm birth seven or more days after an initial course of prenatal corticosteroids with repeat dose(s), compared with no repeat corticosteroid treatment, reduced the risk of their infants experiencing the primary outcomes respiratory distress syndrome (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.75 to 0.91, eight trials, 3206 infants, number needed to treat to benefit (NNTB) 17, 95% CI 11 to 32) and serious infant outcome (RR 0.84, 95% CI 0.75 to 0.94, seven trials, 5094 infants, NNTB 30, 95% CI 19 to 79).Treatment with repeat dose(s) of corticosteroid was associated with a reduction in mean birthweight (mean difference (MD) -75.79 g, 95% CI -117.63 to -33.96, nine trials, 5626 infants). However, outcomes that adjusted birthweight for gestational age (birthweight Z scores, birthweight multiples of the median and small-for-gestational age) did not differ between treatment groups.At early childhood follow-up, no statistically significant differences were seen for infants exposed to repeat prenatal corticosteroids compared with unexposed infants for the primary outcomes (total deaths; survival free of any disability or major disability; disability; or serious outcome) or in the secondary outcome growth assessments. In women, for the two primary outcomes, there was no increase in infectious morbidity of chorioamnionitis or puerperal sepsis, and the likelihood of a caesarean birth was unchanged.
Authors' conclusions: The short-term benefits for babies of less respiratory distress and fewer serious health problems in the first few weeks after birth support the use of repeat dose(s) of prenatal corticosteroids for women still at risk of preterm birth seven days or more after an initial course. These benefits were associated with a small reduction in size at birth. The current available evidence reassuringly shows no significant harm in early childhood, although no benefit.Further research is needed on the long-term benefits and risks for the woman and baby. Individual patient data meta-analysis may clarify how to maximise benefit and minimise harm.
Conflict of interest statement
CAC and JEH are investigators in the Australasian Collaborative Trial of Repeat Doses of Corticosteroid for the Prevention of Neonatal Respiratory Disease (Crowther 2006).
Figures
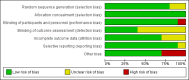
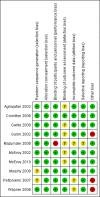






























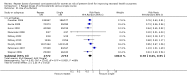






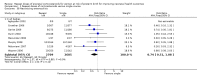




















































Update of
-
Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes.Cochrane Database Syst Rev. 2011 Jun 15;(6):CD003935. doi: 10.1002/14651858.CD003935.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jul 05;(7):CD003935. doi: 10.1002/14651858.CD003935.pub4. PMID: 21678343 Free PMC article. Updated.
References
References to studies included in this review
Aghajafari 2002 {published data only}
-
- Aghajafari F, Murphy K, Ohlsson A, Amankwah K, Matthews S, Hannah M. Multiple versus single courses of antenatal corticosteroids for preterm birth: a pilot study. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(4):321‐9. - PubMed
Crowther 2006 {published data only}
-
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DK, Hiller JE, et al. Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:861–7. - PubMed
-
- Battin M, Bevan C, Harding J. Repeat doses of antenatal steroids and hypothalamic‐pituitary‐adrenal axis (HPA) function. American Journal of Obstetrics and Gynecology 2007;197:40.e1‐40.e6. - PubMed
-
- Battin M, Bevan C, Harding J. Growth in the neonatal period after repeat courses of antenatal corticosteroids: data from the ACTORDS randomised trial. Archives of Disease in Childhood Fetal & Neonatal Edition 2012;97(2):F99‐F105. - PubMed
-
- Battin MR, Bevan C, Morton SM, Harding JE. Repeat courses of antenatal corticosteroids do not alter hypothalamic‐pituitary‐adrenal axis function after birth; results of a randomised controlled trial. Pediatric Academic Societies Annual Meeting; 2004 May 1‐4; San Francisco, USA. 2004.
-
- Crosby DD, Ashwood PJ, Khong TY, Crowther CA. Effects of repeat dose prenatal corticosteroids on placental pathology: results from the ACTORDS trial. Journal of Paediatrics and Child Health 2011;47(Suppl 1):53.
Garite 2009 {published data only}
-
- Garite T, Kurtzman J, Maurel K, Clark R, for the Obstetrix Collaborative Research Network. Impact of a 'rescue course' of antenatal corticosteroids: a multicenter randomized placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2009;200:248.e1‐248.e9. - PubMed
-
- Kurtzman J, Garite T, Clark R, Maurel K, The Obstetrix Collaborative Research Network. Impact of a "rescue course" of antenatal corticosteroids (ACS): a multicenter randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S2. - PubMed
-
- Obstetrix Medical Group. A randomized trial comparing the impact of one versus two courses of ACS on neonatal outcome. ClinicalTrials.gov (www.clinicaltrials.gov) (accessed 20 February 2008).
Guinn 2002 {published data only}
-
- Guinn D, Atkinson M, Sullivan L, Lee M, MacGregor S, Parilla B, et al. Single versus weekly courses of antenatal corticosteroids for women at risk of preterm delivery: a randomized controlled trial. Obstetrics & Gynecology 2003;101(1):195.
-
- Guinn D, BMZ Study Group. Multicenter randomized trial of single versus weekly courses of antenatal corticosteroids (ACS). American Journal of Obstetrics and Gynecology 2001;184(1):S6.
-
- Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla B, et al. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery. JAMA 2001;286(13):1581‐7. - PubMed
-
- Guinn DA, BMZ Study Group. Multicenter randomized trial of single versus weekly courses of antenatal corticosteroids (ACS): interim analysis. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S12.
-
- Lee M, Davies J, Atkinson MW, Guinn D, BMZ Study Group. Efficacy of weekly courses of antenatal corticosteroids (ACS) in preterm premature rupture of the membranes. American Journal of Obstetrics and Gynecology 2001;184(1):S8. - PubMed
Mazumder 2008 {published data only}
-
- Mazumder P, Dutta S, Kaur J, Narang A. Single versus multiple courses of antenatal betamethasone and neonatal outcome: a randomized controlled trial. Indian Pediatrics 2008;45:650‐2. - PubMed
McEvoy 2002 {published data only}
-
- McEvoy C. Effects of single versus weekly courses of antenatal steroids (AS) on functional residual capacity in preterm infants: a randomized trial. Pediatric Research 2001;49(4):388A. - PubMed
-
- McEvoy C, Bowling S, Williamson K, Lozano D, Tolaymat L, Collins J, et al. Effects of single versus weekly courses of antenatal steroids (AS) on functional residual capacity in preterm infants: a randomized trial. Pediatric Academic Societies Annual Meeting; 2001 April 28‐May 1; Baltimore Convention Centre, Baltimore, Maryland, USA. 2001:Abstract no: 2228.
-
- McEvoy C, Bowling S, Williamson R, Lozano D, Tolaymat L, Izquierdo L, et al. The effect of a single remote course versus weekly courses of antenatal corticosteroids on functional residual capacity in preterm infants: a randomized trial. Pediatrics 2002;110:280‐4. - PubMed
McEvoy 2010 {published data only}
-
- McEvoy C, Schilling D, Clay N, Spitale P, Durand M. Neurodevelopmental outcome and growth in infants randomized to a single rescue course of antenatal steroids. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3829.270.
-
- McEvoy C, Schilling D, Spitale P, Gravett M, Durand M. Pulmonary function and respiratory outcomes at 12‐24 months in preterm infants randomized to a single rescue course of antenatal steroids. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
-
- McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada. 2007.
Murphy 2008 {published and unpublished data}
-
- Asztalos E, Murphy K, Hannah M, Willan A, MACS Collaborative Group. Outcomes of children at two years after multiple courses of antenatal corticosteroids for threatened preterm birth: the multiple antenatal corticosteroids study (MACS). Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
-
- Asztalos EV, Murphy KE, Hannah ME, Willan AR, Matthews SG, Ohlsson A, et al. Multiple courses of antenatal corticosteroids for preterm birth study: 2‐year outcomes. Pediatrics 2010;126:e1045‐e1055. - PubMed
-
- Maternal Infant and Reproductive Health Research Unit. Multiple courses of antenatal steroids for preterm birth (MACS). Center for Mother, Infant, and Child Research (www.utoronto.ca/miru/macs/protocol02.htm) 2002.
-
- Murphy K, Asztalos E, Hannah M, Willan A, Hewson S, Ohlsson A, et al. Multiple courses of antenatal corticosteroids for preterm birth study (MACS): results of the three month maternal post partum questionnaire. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S173.
-
- Murphy K, Hannah M, Willan A, Hewson S, Ohlsson A, Kelly E, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet 2008;372:2143‐51. - PubMed
Peltoniemi 2007 {published data only}
-
- Janer C, Helve O, Pitkänen OM, Kari MA, Peltoniemi OM, Hallman M, et al. Expression of airway epithelial sodium channel in the preterm infant is related to respiratory distress syndrome but unaffected by repeat antenatal beta‐methasone. Neonatology 2010;97(2):132‐8. - PubMed
-
- Koivisto M, Peltoniemi OM, Saarela T, Tammela O, Jouppila P, Hallman M. Blood glucose level in preterm infants after antenatal exposure to glucocorticoid. Acta Paediatrica 2007;96(5):664‐8. - PubMed
-
- Peltoniemi O, Kari, M, Lano A, Yliherva A, Puosi R, Lehtonen L, et al. Two‐year follow‐up of a randomized trial with repeated antenatal betamethasone. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(6):F402‐F406. - PubMed
-
- Peltoniemi OM. 2‐year follow‐up of randomized trial on a single repeat dose of antenatal betamethasone in imminent preterm birth. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2008 May 2‐6; Honolulu, Hawaii. 2008.
-
- Peltoniemi OM, Kari MA, Halmesmaki E, Raudaskoski T, Tammela O, Uotila J, et al. The effect of second course of antenatal betamethasone (BM) on neonatal morbidity in preterm infants: a randomised trial. Pediatric Research 2005;58(2):404.
Wapner 2006 {published data only}
-
- Church M. Auditory brainstem responses (ABRs) in neonates exposed to repeated courses of antenatal corticosteroids. Program of the Twenty Eighth Annual Midwinter Research Meeting of the Association for Research in Otolaryngology; 2005 Feb 19‐24; New Orleans, Louisiana. 2005:Abstract no: 669.
References to studies excluded from this review
Bontis 2011 {published data only}
-
- Bontis N, Vavilis D, Tsolakidis D, Goulis DG, Tzevelekis P, Kellartzis D, et al. Comparison of single versus multiple courses of antenatal betamethasone in patients with threatened preterm labor. Clinical and Experimental Obstetrics and Gynecology 2011;38(2):165‐7. - PubMed
Mercer 2001 {published data only}
-
- Mercer B, Egerman R, Beazley D, Sibai B, Carr T, Sepesi J. Steroids reduce fetal growth: analysis of a prospective trial. American Journal of Obstetrics and Gynecology 2001;184(1):S7.
-
- Mercer B, Egerman R, Beazley D, Sibai B, Carr T, Sepesi J. Weekly antenatal steroids trial in women at risk of preterm birth: a randomized trial. American Journal of Obstetrics and Gynecology 2001;184(1):S6.
-
- Sawady J, Mercer B. Impact of repeated doses of antenatal corticosteroids on placental growth and histology. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S73. - PubMed
Romejko‐Wolniewicz 2013 {published data only}
-
- Romejko‐Wolniewicz E, Oleszczuk L, Zareba‐Szczudlik J, Czajkowski K. Dosage regimen of antenatal steroids prior to preterm delivery and effects on maternal and neonatal outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2013;26(3):237‐41. - PubMed
Thorp 2000 {published data only}
-
- Thorp JA, Yeast JD, Cohen GR, Wickstrom EA, D' Angelo LJ. Repeated antenatal betamethasone and perinatal outcome. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S21.
References to studies awaiting assessment
Atarod 2014 {published data only}
-
- Atarod Z, Taghipour M, Roohanizadeh H, Fadavi S, Taghavipour M. Effects of single course and multicourse betamethasone prior to birth in the prognosis of the preterm neonates: a randomized, double‐blind placebo‐control clinical trial study. Journal of Research in Medical Sciences 2014;19(8):715‐9. - PMC - PubMed
-
- Ataroud Z. Single versus multiple courses of antenatal betamethasone: evaluation of preterm infant's outcome. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010). IRCT138711261689N1 2010.
Crowther 2011 {published data only}
-
- Crowther C, Doyle LW, Anderson P, Harding JE, Haslam RR, Hiller JE. Repeat dose(s) of prenatal corticosteroids for women at risk of preterm birth: early school‐age outcomes (6 to 8 years) for the ACTORDS trial. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3123.6.
-
- Crowther CA, Doyle LW, Anderson P, Harding JE, Haslam RR, Hiller JE, et al. Repeat dose(s) of prenatal corticosteroids for women at risk of preterm birth: Early school‐age outcomes (6 to 8 years) for children in the ACTORDS trial. Journal of Paediatrics and Child Health 2011;47(Suppl 1):52‐3.
-
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics 2015;135(2):e405‐e415. - PubMed
-
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA. Cardiovascular risk factors after exposure to repeat antenatal betamethasone: early school‐age follow‐up of a randomised trial (ACTORDS). Journal of Paediatrics and Child Health 2011;47(Suppl 1):20.
-
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA. Repeat antenatal betamethasone does not affect basal salivary cortisol at early school‐age: a randomised controlled trial (ACTORDS). Journal of Paediatrics and Child Health 2011;47(Suppl 1):19.
Murphy 2012 {published data only}
-
- Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS‐5). BMC Pregnancy and Childbirth 2014;14(1):272. - PMC - PubMed
-
- Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al. Multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS‐5): association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years of age (MACS‐5). American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S321‐S322. - PMC - PubMed
-
- Asztalos EV, Murphy KE, Willan AR, Matthews SG, Ohlsson A, Saigal S, et al. for the MACS‐5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: Outcomes in children at 5 years of age (MACS‐5). Journal of the American Medical Association Pediatrics 2013;167(12):1102‐10. - PubMed
-
- Murphy KE, Willan AR, Hannah ME, Ohlsson A, Kelly EN, Matthews SG, et al. Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstetrics & Gynecology 2012;119(5):917‐23. - PubMed
References to ongoing studies
Sobhrabvand 2001 {published data only}
-
- Sohrabvand F, Behbahani B, Kazeminejad A. Effects of single versus multiple courses of corticosteroid therapy on pregnancy results in women with PPROM. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):528.
TEAMS 1999 {published and unpublished data}
-
- Adams H. Trial of the effects of antenatal multiple courses of steroids versus a single course (TEAMS): pilot study. Current Controlled Trials (http://www.controlled‐trials.com) (accessed 4 November 2003) 2003.
Additional references
Ashwood 2006
-
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DJ, Hiller JE, et al. Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:861‐7. - PubMed
Benediktsson 1993
-
- Benediktsson R, Lindsay RS, Noble J, Secki JR, Edwards CRW. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 1993;341:339‐41. - PubMed
Brownfoot 2013
Crowther 2012
-
- Crowther C, Aghajafari F, Askie L, Asztalos E, Brocklehurse P, Bubner T, et al. Repeat prenatal corticosteroid prior to preterm birth: a systematic review and individual participant data meta‐analysis for the PRECISE study group (prenatal repeat corticosteroid international IPD study group: assessing the effects using the best level of evidence) ‐ study protocol. Systematic Reviews 2012;1(1):12. - PMC - PubMed
Dalziel 2005a
-
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30‐year follow‐up of a randomised controlled trial. Lancet 2005;365(9474):1856‐62. - PubMed
Dalziel 2005b
Dunlop 1997
-
- Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. Journal of Maternal‐Fetal Medicine 1997;6:309‐13. - PubMed
Esplin 2000
-
- Esplin M, Fausett M, Smith S, Oshiro B, Porter TF, Branch DW, et al. Multiple courses of antenatal steroids associated with a delay in long‐term psychomotor development in children with birth weight < 1500 grams. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S24.
Fowden 1996
-
- Fowden AL, Szemere J, Hughes RS, Forhead AJ. The effects of cortisol on the growth rate of the sheep fetus during late gestation. Journal of Endocrinology 1996;151:97‐105. - PubMed
French 1998
-
- French N, Hagan R, Evans S, Godfrey M, Newnham J. Repeated antenatal corticosteroids: behaviour outcomes in a regional population of very preterm infants. Pediatric Research 1998;43:214A.
French 1999
-
- French N, Hagan R, Evans S, Godfrey M, Newnham J. Repeated antenatal corticosteroids: size at birth and subsequent development. American Journal of Obstetrics and Gynecology 1999;180:114‐21. - PubMed
French 2004
-
- French N, Hagan R, Evans S, Mullan A, Newnham J. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behaviour. American Journal of Obstetrics and Gynecology 2004;190:588‐95. - PubMed
Hasbargen 2001
-
- Hasbargen U, Reber D, Versmold H, Schulze A. Growth and development of children to 4 years of age after repeated antenatal steroid administration. European Journal of Pediatrics 2001;160:552‐5. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ikegami 1997
-
- Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. American Journal of Respiratory and Critical Care Medicine 1997;156:178‐84. - PubMed
Jensen 2002
-
- Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late gestation fetal sheep. Journal of Endocrinology 2002;174:27‐36. - PubMed
Jobe 1994
-
- Jobe AH. National Institutes of Health. Report of the Consensus Development Conference on the effect of corticosteroids for fetal lung maturation on perinatal outcomes. Bethesda, MD: US Department of Health and Human Services, Public Health Service, 1994:39‐41.
Johnson 1993
Laws 2005
-
- Laws PJ, Sullivan EA. Australia's mothers and babies 2003. Perinatal Statistics Series no. 16. Sydney: Australian Institute of Health and Welfare, National Perinatal Statistics Unit, 2005.
Liggins 1972
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515‐25. - PubMed
McKenna 2000
-
- McKenna DS, Witter GM, Nagaraja HN, Samuels P. The effects of repeat doses of antenatal corticosteroids on maternal adrenal function. American Journal of Obstetrics and Gynecology 2000;183:669‐73. - PubMed
McLaughlin 2003
-
- McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:101‐6. - PubMed
Mildenhall 2006
Moise 1995
-
- Moise AA, Wearden ME, Kozinetz CA, Gest AL, Welty SE, Hansen TN. Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics 1995;95:845‐50. - PubMed
Newnham 1999
-
- Newnham JP, Evans S, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. Journal of Maternal‐Fetal Medicine 1999;8(3):81‐7. - PubMed
NIH 1995
-
- NIH Consensus Development Panel. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995;273:413‐8. - PubMed
NIH 2000
-
- NIH Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses. National Institutes of Health Consensus development conference statement. Obstetrics & Gynecology 2000;98:144‐50. - PubMed
Padbury 1996
-
- Padbury JF, Ervin G, Polk D. Extrapulmonary effects of antenatally administered steroids. Journal of Pediatrics 1996;128:167‐72. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
Rojas‐Reyes 2012
Seckl 2004
-
- Seckl JR, Meaney MJ. Glucocorticoid programming. Annals of the New York Academy of Sciences 2004;1032:63‐84. - PubMed
Thorp 2002
-
- Thorp JA, Etzenhouser J, O'Connor M, Jones A, Jones P, Belden B, et al. Effects of phenobarbital and multiple‐dose antenatal/postnatal steroid on developmental outcome at age 7 years. American Journal of Obstetrics and Gynecology 2002;185(6):S87. - PubMed
Tschanz 1995
-
- Tschanz SA, Danke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biology of the Neonate 1995;68:229‐45. - PubMed
References to other published versions of this review
Crowther 2000
Crowther 2007
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

