The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1
- PMID: 26144316
- PMCID: PMC4506698
- DOI: 10.1016/j.cell.2015.06.017
The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1
Abstract
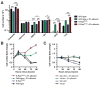
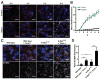
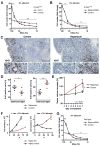
Despite being surrounded by diverse nutrients, mammalian cells preferentially metabolize glucose and free amino acids. Recently, Ras-induced macropinocytosis of extracellular proteins was shown to reduce a transformed cell's dependence on extracellular glutamine. Here, we demonstrate that protein macropinocytosis can also serve as an essential amino acid source. Lysosomal degradation of extracellular proteins can sustain cell survival and induce activation of mTORC1 but fails to elicit significant cell accumulation. Unlike its growth-promoting activity under amino-acid-replete conditions, we discovered that mTORC1 activation suppresses proliferation when cells rely on extracellular proteins as an amino acid source. Inhibiting mTORC1 results in increased catabolism of endocytosed proteins and enhances cell proliferation during nutrient-depleted conditions in vitro and within vascularly compromised tumors in vivo. Thus, by preventing nutritional consumption of extracellular proteins, mTORC1 couples growth to availability of free amino acids. These results may have important implications for the use of mTOR inhibitors as therapeutics.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Comment in
-
mTORC1 maintains metabolic balance.Cell Res. 2015 Oct;25(10):1085-6. doi: 10.1038/cr.2015.107. Epub 2015 Sep 11. Cell Res. 2015. PMID: 26358187 Free PMC article.
References
-
- Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. International journal of medical microbiology : IJMM. 2002;291:487–494. - PubMed
-
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science (New York, NY) 1986;233:1061–1068. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews Cancer. 2011;11:85–95. - PubMed
-
- Cybulski N, Zinzalla V, Hall MN. Inducible raptor and rictor knockout mouse embryonic fibroblasts. Methods in molecular biology (Clifton, NJ) 2012;821:267–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5P50CA101955-07/CA/NCI NIH HHS/United States
- 1U10CA180944-01/CA/NCI NIH HHS/United States
- R01 CA201318/CA/NCI NIH HHS/United States
- R01 CA190092/CA/NCI NIH HHS/United States
- 1R01CA190092-01/CA/NCI NIH HHS/United States
- P50 CA101955/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- 5U01CA168409-3/CA/NCI NIH HHS/United States
- 5P30CA45508-26/CA/NCI NIH HHS/United States
- P01 CA104838/CA/NCI NIH HHS/United States
- U10 CA180944/CA/NCI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- U01 CA168409/CA/NCI NIH HHS/United States
- 5P30CA045508/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

